Article Text
Abstract
Introduction Since sex-based biological and gender factors influence COVID-19 mortality, we wanted to investigate the difference in mortality rates between women and men in sub-Saharan Africa (SSA).
Method We included 69 580 cases of COVID-19, stratified by sex (men: n=43 071; women: n=26 509) and age (0–39 years: n=41 682; 40–59 years: n=20 757; 60+ years: n=7141), from 20 member nations of the WHO African region until 1 September 2020. We computed the SSA-specific and country-specific case fatality rates (CFRs) and sex-specific CFR differences across various age groups, using a Bayesian approach.
Results A total of 1656 deaths (2.4% of total cases reported) were reported, with men accounting for 70.5% of total deaths. In SSA, women had a lower CFR than men (mean  = −0.9%; 95% credible intervals (CIs) −1.1% to −0.6%). The mean CFR estimates increased with age, with the sex-specific CFR differences being significant among those aged 40 years or more (40–59 age group: mean
= −0.9%; 95% credible intervals (CIs) −1.1% to −0.6%). The mean CFR estimates increased with age, with the sex-specific CFR differences being significant among those aged 40 years or more (40–59 age group: mean  = −0.7%; 95% CI −1.1% to −0.2%; 60+ years age group: mean
= −0.7%; 95% CI −1.1% to −0.2%; 60+ years age group: mean  = −3.9%; 95% CI −5.3% to −2.4%). At the country level, 7 of the 20 SSA countries reported significantly lower CFRs among women than men overall. Moreover, corresponding to the age-specific datasets, significantly lower CFRs in women than men were observed in the 60+ years age group in seven countries and 40–59 years age group in one country.
= −3.9%; 95% CI −5.3% to −2.4%). At the country level, 7 of the 20 SSA countries reported significantly lower CFRs among women than men overall. Moreover, corresponding to the age-specific datasets, significantly lower CFRs in women than men were observed in the 60+ years age group in seven countries and 40–59 years age group in one country.
Conclusions Sex and age are important predictors of COVID-19 mortality globally. Countries should prioritise the collection and use of sex-disaggregated data so as to design public health interventions and ensure that policies promote a gender-sensitive public health response.
- COVID-19
- epidemiology
Data availability statement
Data are available on reasonable request. The data (data dictionary, statistical code) that support the findings of this study are available upon reasonable request from the corresponding author. Some of the data are publicly available through situation reports produced by Ministries of Health and WHO/AFRO on their respective websites.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Key questions
What is already known?
Sex-based biological as well as gender factors contribute to the variation in COVID-19 mortality between men and women.
In most of the non-African countries, sex-specific COVID-19 severity and mortality were substantially worse for men than for women, during the first wave of the COVID-19 pandemic.
Data on COVID-19 cases and deaths, disaggregated by both age and sex from the WHO African region, has been scarce.
What are the new findings?
In sub-Saharan Africa (SSA), overall, men had a higher case fatality rate (CFR) than women. When disaggregated by age, this difference persisted only in individuals aged 40 years or more.
7 among the 20 SSA countries included in this study also reported significantly higher CFRs in men than women for the age-aggregated dataset.
Corresponding to the country-wise age-specific datasets, we found significantly lower CFRs in women than men in the 60+ years age group in seven countries and in the 40–59 years age group in one country.
What do the new findings imply?
Both sex and age are important predictors of COVID-19 mortality in SSA, similar to other regions.
Public health prevention activities and responses should take into account gender differences in terms of disease severity and mortality, especially among men aged 40 years or more in SSA.
Introduction
SARS-CoV-2, first reported in December 2019, rapidly underwent an exponential increase in cases and related fatalities, affecting almost every country in the world. Increasing evidence has demonstrated that infections caused by SARS-CoV-2 lead to major variations in disease severity and mortality between women and men.1 During the first wave of the COVID-19 pandemic in the USA, China, Singapore, South Korea and multiple European countries, sex-specific COVID-19 susceptibility, severity and mortality were substantially worse for men than for women.2 Furthermore, globally, men demonstrate increased mortality due to SARS-CoV-2 infection compared with women.3 4 The difference in the fatality of COVID-19 could be attributed to sex-based biological (immune response, hormones and genetics) and/or gender factors (social, behavioural or lifestyle aspects).1 5 6 Hence, the mortality rate difference between men and women could vary across regions, and this question is particularly interesting in the context of Africa, where women are disproportionately affected by resource limitedness, restricted civil liberty, disparity in access to education and health service, gender-based violence, gender norms and roles,2 and diseases such as HIV.7
While COVID-19 had approximately infected 25 000 000 individuals and caused 840 000 deaths globally, resulting in a case fatality rate (CFR) of around 3.4% as of 31 August 2020, Africa (representing ~17% of the world’s population) accounted for 4% of total confirmed cases and 3% of the total deaths reported, resulting in a CFR of around 2.1%, much lower than the global estimate,8 making it one of the least affected continents,9 for which there are various explanations.10–12 Furthermore, the availability of data on COVID-19 cases and deaths that are disaggregated by both age and sex from the WHO African region has been particularly scarce. As per the report published by Global Health 5050 in January 2021, the total number of COVID-19 cases disaggregated by sex in the region accounted for merely 2.5% of total cases and 2.7% of total deaths reported globally.13 Moreover, among the countries with sex information on cases, only 36% shared age information, whereas among the countries with sex information on deaths, only 42% shared age-disaggregated data.14
Since there is little published research on the impact of COVID-19 among different sexes and age groups in sub-Saharan Africa (SSA), our objective was to estimate COVID-19 mortality rates, as determined by crude CFR, among men and women within 20 SSA countries, from the beginning of the pandemic to early September 2020. According to the Sex, Gender and COVID-19 Health Policy Portal (it reviews national COVID-19 public health policies that adopt a gender-responsive approach globally),15 few policies and public health responses to COVID-19 take gender and sex into account, despite the considerable roles of both in transmission, course and outcome of infectious diseases, such as Ebola, dengue and severe acute respiratory syndrome. Sex-disaggregated and age-disaggregated data are essential to understand the course of the pandemic, identify existing or arising gender-related health inequities and the subsequent formations of gender-sensitive responses to the pandemic.16
Methods
Study design and settings
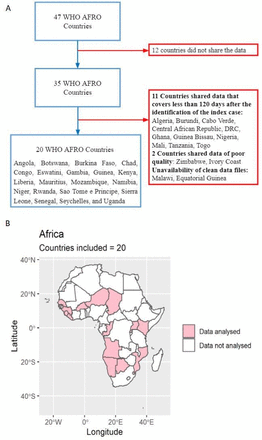
We carried out a cross-sectional analysis of the COVID-19 cases diagnosed from the date of first infection (index case) until 1 September 2020. Although the index case date varied across countries (table 1), we assumed that the mortality rate difference between women and men would mostly remain unaffected by this variation. Our primary data sources were national situation reports made public by all SSA countries experiencing the COVID-19 pandemic. The data from each country report were extracted and merged into a linelist. Among the 47 member states comprising the WHO African region, 35 shared sex-disaggregated and age-disaggregated data with the WHO. We included countries that shared data for at least 120 days from the identification of the index case, in order to mitigate biases due to delayed reporting of either cases or deaths.17 Twenty countries, Angola, Botswana, Burkina Faso, Chad, Congo, Eswatini, Gambia, Guinea, Kenya, Liberia, Mauritius, Mozambique, Namibia, Niger, Rwanda, São Tomé e Principe, Sierra Leone, Senegal, Seychelles and Uganda, met the criteria and were included (figure 1). Among these 20, we only included the cases with confirmed information about sex, age, patient’s outcome status and date of reporting. The time period covered for each country is shown in table 1.
(A) Flow chart illustrating the inclusion and exclusion criteria used to select countries; (B) Map showing the 20 member states from WHO African region that were included in the final analysis.
Sample characteristics of the data included in this study
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Variables
For each country, we considered sex (women and men), age, date of reporting and clinical outcomes (dead and alive). The alive category encompassed patients who were still alive at the time of reporting: this included recovered cases. We classified the cases into three age categories: 0–39, 40–59 and 60+ years.
Statistical analysis
We followed a Bayesian approach to compute CFRs as the posterior probabilities, since it offers a robust inferential framework that accurately estimates the probability of rare events. This allows us to be explicit in our prior belief of observing no gender differences in CFR, making the detected differences even more relevant. It is important to note that we are not looking just at the case counts, but at the proportion of deaths to non-deaths. Thus, observing 0 deaths is as informative as any other number of deaths to estimate the probability of dying. Hence, we included countries that also demonstrated a low number of deaths. We used non-informative priors, since age-specific CFRs from the SSA region were not available. We modelled deaths as a binomial random variable, Binomial(N, p), with p being the ratio of deaths to cases and N being the number of confirmed cases. Thus, the parameter p represents the CFR, to which was attributed a non-informative Beta(α = β = 0.33) prior distribution, first proposed by Kerman.18 For each country as well as for the 20 countries combined, we computed the estimates for sex-specific and age-specific CFRs, overall (all age groups combined) CFRs and the crude CFR differences  ,
,

with a negative  indicating that CFR was higher in men than in women.
indicating that CFR was higher in men than in women.
To compute the Bayesian estimates, we employed the Markov chain Monte Carlo (MCMC) sampling algorithm, No U-Turn Sampler (NUTS),19 that was implemented in the probabilistic programming package PyMC3.20 We used the NUTS, with four parallel chains, with a sample size of 5000 per chain, after 1000 burn-in samples. Moreover, to evaluate the convergence of MCMC, we generated four independent Markov chains and the resulting marginal distributions were compared using the Gelman-Rubin diagnostic, a ratio that should not exceed 1.2 for convergence.21 22 We used the strict criterion in our model that this diagnostic <1.1.
For each Bayesian estimate, we reported the corresponding posterior beta distributions along with their mean and the 95% highest posterior density (HPD) intervals.
Sensitivity analysis: To assess the robustness of our prior, we performed the same analysis using two more priors based on the binomial likelihood: Beta(1,1) or Bayes-Laplace prior23 and Beta(0.5, 0.5) or Jeffreys prior.24
Results
SSA estimates
Sample characteristics of the COVID-19 cases and deaths: as of 1 September 2020, a total of 69 580 COVID-19 cases and 1656 (2.4% of total cases; 1656/69 580) COVID-19 related deaths were reported by the 20 member states in the WHO African region included in our research (figure 1B). Men accounted for 61.9% (43 071/69 580) of the total reported cases and 70.5% (1168/1656) of total deaths. A percentage of 51.3 (850/1656) of the total deaths occurred in the ‘60+’ age group. Among the confirmed cases of COVID-19, the median age of those still alive was 35 years, while it was 60 years for individuals who died, at the time of data collection (table 1).
Mean CFR estimates in men and women: mean CFR estimates increased with age for both men and women (figure 2 and online supplemental table S1).
Supplemental material
SSA-specific estimates, after merging the data from 20 SSA countries: posterior distributions of CFR in women, CFR in men and  (=CFR in women – CFR in men), for each age group and all age groups combined. The horizontal black line represents the 95% HPDs. Ninety-five per cent HPD intervals are the same as that of 95% HDIs. Ninety-five per cent HPDs for
(=CFR in women – CFR in men), for each age group and all age groups combined. The horizontal black line represents the 95% HPDs. Ninety-five per cent HPD intervals are the same as that of 95% HDIs. Ninety-five per cent HPDs for  do not contain 0 for estimates obtained in both 40–59 and 60+ years age groups and overall (all age groups combined), indicating significant differences between sex-specific CFRs. CFRs, case fatality rates; HDI, highest density interval; HPD, highest posterior density; SSA, sub-Saharan Africa.
do not contain 0 for estimates obtained in both 40–59 and 60+ years age groups and overall (all age groups combined), indicating significant differences between sex-specific CFRs. CFRs, case fatality rates; HDI, highest density interval; HPD, highest posterior density; SSA, sub-Saharan Africa.
Risk differences,  : Overall, CFR was significantly higher in men than in women (mean
: Overall, CFR was significantly higher in men than in women (mean  = −0.9%; 95% HPD intervals −1.1% to −0.6%). When analysing the data by age, both the ‘40–59’ (mean
= −0.9%; 95% HPD intervals −1.1% to −0.6%). When analysing the data by age, both the ‘40–59’ (mean  = −0.7%; 95% HPD intervals −1.1% to −0.2%) and ‘60+’ (mean
= −0.7%; 95% HPD intervals −1.1% to −0.2%) and ‘60+’ (mean  = −3.9%; 95% HPD intervals −5.3% to −2.4%) years age groups demonstrated significantly higher CFRs for men than for women. There were no significant differences between sex-specific CFRs in the ‘0–39’ years age group (mean
= −3.9%; 95% HPD intervals −5.3% to −2.4%) years age groups demonstrated significantly higher CFRs for men than for women. There were no significant differences between sex-specific CFRs in the ‘0–39’ years age group (mean 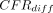 = −0.1%; 95% HPD intervals −0.2% to 0.1%) (figure 2 and online supplemental table S1).
= −0.1%; 95% HPD intervals −0.2% to 0.1%) (figure 2 and online supplemental table S1).
Country-specific estimates
Sample characteristics of the COVID-19 cases and deaths: among the 20 countries included in this study, the countries with the highest number of cases were Guinea (14.8%, n=10 278), Congo (13.6%, n=9489), Senegal (13.0%, n=9039), Namibia (10.6%, n=7407) and Kenya (9.9%, n=6869). Congo accounted for the largest proportion of all deaths reported (24.6%, n=407), followed by Kenya (17.9%, n=296; table 1).
Mean CFR estimates in women and men: crude mean CFR estimates ranged from 0.2% (0.1% to 0.4%) in Guinea to 9.2% (5.5% to 13.0%) in Chad among women, and from 0 in Botswana to 10.5% (8.3% to 12.9%) in Chad among men (online supplemental table S2). Mean crude CFR estimates increased with age, except for Angola, Mauritius, São Tomé e Principe and Seychelles (online supplemental figure S2 and table S2).
Risk differences: 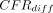 : after combining the data from all the age groups, CFRs were significantly higher in men than women in seven SSA countries: Angola (mean
: after combining the data from all the age groups, CFRs were significantly higher in men than women in seven SSA countries: Angola (mean 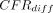 = −1.8%; 95% HPD intervals −3.4% to −0.1%), Eswatini (mean
= −1.8%; 95% HPD intervals −3.4% to −0.1%), Eswatini (mean 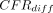 = −1.3%; 95% HPD intervals −2.1% to −0.5%), Guinea (mean
= −1.3%; 95% HPD intervals −2.1% to −0.5%), Guinea (mean  = −0.7%; 95% HPD intervals −0.9% to −0.4%), Kenya (mean
= −0.7%; 95% HPD intervals −0.9% to −0.4%), Kenya (mean  = −1.4%; 95% HPD intervals −2.4% to −0.4%), Mauritius (mean
= −1.4%; 95% HPD intervals −2.4% to −0.4%), Mauritius (mean  = −3.3%; 95% HPD intervals −6.6% to −0.3%), Senegal (mean
= −3.3%; 95% HPD intervals −6.6% to −0.3%), Senegal (mean  = −0.9%; 95% HPD intervals −1.4% to −0.4%) and Sierra Leone (mean
= −0.9%; 95% HPD intervals −1.4% to −0.4%) and Sierra Leone (mean  = −3.8%; 95% HPD intervals −6.4% to −1.3%) (figure 3 & online supplemental table S3).
= −3.8%; 95% HPD intervals −6.4% to −1.3%) (figure 3 & online supplemental table S3).
Country-specific estimates, all age groups combined: posterior distributions of  (=CFR in women – CFR in men), for each country. The horizontal black line represents the 95% HPDs. Ninety-five per cent HPD intervals are the same as that of 95% HDIs. Ninety-five per cent HPDs for Angola (−0.034 to −0.001), Eswatini (−0.021 to −0.005), Guinea (−0.009 to –0.004), Kenya (−0.024 to −0.004), Mauritius (−0.066 to −0.003), Senegal (−0.014 to −0.004) and Sierra Leone (−0.064 to −0.013) do not contain 0, indicating significant differences between sex-specific CFRs in these countries. CFRs, case fatality rates; HDI, highest density interval; HPD, highest posterior density.
(=CFR in women – CFR in men), for each country. The horizontal black line represents the 95% HPDs. Ninety-five per cent HPD intervals are the same as that of 95% HDIs. Ninety-five per cent HPDs for Angola (−0.034 to −0.001), Eswatini (−0.021 to −0.005), Guinea (−0.009 to –0.004), Kenya (−0.024 to −0.004), Mauritius (−0.066 to −0.003), Senegal (−0.014 to −0.004) and Sierra Leone (−0.064 to −0.013) do not contain 0, indicating significant differences between sex-specific CFRs in these countries. CFRs, case fatality rates; HDI, highest density interval; HPD, highest posterior density.
In the ‘60+’ years age group, CFRs were significantly higher in men in seven countries: Eswatini (mean  = −9.4%; 95% HPD intervals −17.0% to −2.1%), Guinea (mean
= −9.4%; 95% HPD intervals −17.0% to −2.1%), Guinea (mean  = −4.2%; 95% HPD intervals −6.3% to −2.2%), Kenya (mean
= −4.2%; 95% HPD intervals −6.3% to −2.2%), Kenya (mean  = −10.3%; 95% HPD intervals −18.2% to −2.7%), Mauritius (mean
= −10.3%; 95% HPD intervals −18.2% to −2.7%), Mauritius (mean  = −15.4%; 95% HPD intervals −29.6% to −2.3%), São Tomé e Principe (mean
= −15.4%; 95% HPD intervals −29.6% to −2.3%), São Tomé e Principe (mean 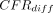 = −6.8%; 95% HPD intervals −13.7% to −0.8%), Senegal (mean
= −6.8%; 95% HPD intervals −13.7% to −0.8%), Senegal (mean  = −4.1%; 95% HPD intervals −6.1% to −2.0%) and Sierra Leone (mean
= −4.1%; 95% HPD intervals −6.1% to −2.0%) and Sierra Leone (mean  = −16.3%; 95% HPD intervals −29.6% to −2.6%) (figure 4 and online supplemental table S3). In the ‘40–59’ years age-group, Angola was the only country that had a significant CFR difference, with men having a higher CFR than women (mean
= −16.3%; 95% HPD intervals −29.6% to −2.6%) (figure 4 and online supplemental table S3). In the ‘40–59’ years age-group, Angola was the only country that had a significant CFR difference, with men having a higher CFR than women (mean  = −2.5%; 95% HPD intervals −4.6% to −0.7%; online supplemental figure S4 and table S3). No country demonstrated significant sex-specific CFR difference in the ‘0–39’ years age group (online supplemental figure S3 and table S3).
= −2.5%; 95% HPD intervals −4.6% to −0.7%; online supplemental figure S4 and table S3). No country demonstrated significant sex-specific CFR difference in the ‘0–39’ years age group (online supplemental figure S3 and table S3).
Country-specific estimates, 60+ years age group: posterior distributions of  (=CFR in women – CFR in men), for each country. The horizontal black line represents the 95% HPDs. Ninety-five per cent HPD intervals are the same as that of 95% HDIs. Ninety-five per cent HPDs for Eswatini (−0.17 to −0.021), Guinea (−0.063 to −0.022), Kenya (−0.182 to −0.027), Mauritius (−0.296 to −0.023), São Tomé e Principe (−0.137 to −0.008), Senegal (−0.061 to −0.02) and Sierra Leone (−0.296 to −0.026) do not contain 0, indicating significant differences between sex-specific CFRs in these countries. CFRs, case fatality rates; HDI, highest density interval; HPD, highest posterior density.
(=CFR in women – CFR in men), for each country. The horizontal black line represents the 95% HPDs. Ninety-five per cent HPD intervals are the same as that of 95% HDIs. Ninety-five per cent HPDs for Eswatini (−0.17 to −0.021), Guinea (−0.063 to −0.022), Kenya (−0.182 to −0.027), Mauritius (−0.296 to −0.023), São Tomé e Principe (−0.137 to −0.008), Senegal (−0.061 to −0.02) and Sierra Leone (−0.296 to −0.026) do not contain 0, indicating significant differences between sex-specific CFRs in these countries. CFRs, case fatality rates; HDI, highest density interval; HPD, highest posterior density.
Sensitivity analysis: our sensitivity analysis using other priors (Bayes-Laplace and Jeffreys) yielded similar estimates as that of Kerman’s prior. Exceptions included countries with scarce data, for example, Seychelles, Botswana and Gambia (online supplemental figure S1).
Discussion
This study provided information on the sex-specific and age-specific difference in SARS-CoV-2 CFR among 20 countries in the SSA until 1 September 2020. As one of the largest studies analysing CFR differences between women and men in SSA, our results showed that age-specific CFRs were higher among men than women in the SSA and that mean CFR differences increased with age.
Our data illustrates that at the regional sub-Saharan level men demonstrate higher crude CFR than women. Our SSA-specific estimates align with most epidemiological global studies where men were reported to have higher mortality than women.4 25–27 Moreover, several papers28 29 showed that being a man places individuals at greater risk for health complications and death. The reasons for the difference between women and men in the fatality of COVID-19 could be attributed to sex-based biological factors and social, behavioural or lifestyle factors related to gender. Several biological determinants like the immune system, genetics, sex hormones and the microbiome could contribute to lower COVID-19 fatality rates among women.5 30 Biological aspects are influenced by gender roles, norms, practices and masculinities which, in turn, further affect health. With regard to gender norms and roles, in most low-income settings men are often the providers of the family and spend most of the time working outside their home, while women are the caregivers that take care of children and house. This division of labour and responsibilities may expose more men than women to COVID-19 infection, preventing the former to follow the social distance measures, thus possibly causing more infections among them although not necessarily increasing the CFR. However, greater predispositions to health-harming behaviours among men (ie, smoking)31 contribute to the development of noncommunicable diseases (NCDs) and health complications later on in life.32 The rising burden of NCDs in developing countries over the past two decades33–35 and the higher impact of NCD risk factors in men than in women36 could lead to increased comorbidity-related mortality among men when paired with COVID-19 infection. In this continent, since HIV prevalence is higher in women than men, women may be more affected by the consequences of the outbreak such as disruption of the supply chain, weakened immune system due to unavailability of ART and, as a consequence, be more susceptible to COVID-19 infection.37 This might explain the low CFR difference in some countries, where higher HIV mortality in women may compensate for higher mortality in men. However, this claim needs to be examined in future studies. Therefore, sex-disaggregated and age-disaggregated data only explain a small fraction of the complex intersectional biological and social health inequities16 that were exposed or have arisen from the COVID-19 outbreak. In order to comprehensively address disparities between women and men, one must also consider the intersecting links of social class, economic conditions, ethnicity, religion and able-bodiedness to biological sex and gender.38
In contrast to such global evidence on the association between sex and COVID-19 CFR, some countries such as India (as of May 2020),39 Nepal, Vietnam and Slovenia (as of 18 September 2020)40 reported lower COVID-19 fatality rates in men than in women. Such differential findings might reflect incomplete COVID-19 data across countries, biases in case identification by sex, inequality in accessing healthcare facilities between women and men or higher infection risks for women in certain countries. Akter41 found that gender inequality in healthcare access, combined with limited health systems capacity, is likely to increase under-reporting of COVID-19 fatalities among the former in the USA (as of 25 July 2020). Gender biases in healthcare lead to incorrect diagnoses and poor treatment for both women and men, subsequently leading to worse health outcomes overall.42 Comprehensively understanding gender biases in healthcare settings are thus needed.
When analysed at the country level, however, only 7 out of 20 SSA countries demonstrated a marked difference in mortality between women and men. A descriptive study from Niger that used sex-disaggregated individual-level data until July 2020 also found no statistical significance in CFR difference between the two groups.43 Out of the 13 countries that did not demonstrate statistically significant CFR differences between women and men, three of them (Botswana, Seychelles and Gambia) had low sample sizes; no significant difference for these country-level samples could thus be attributed to lack of data variance within small samples. Further studies need to be conducted to confirm our findings.
Elderly populations may be disproportionately affected by COVID-19 owing to fragility due to ageing and physiological changes, weaker immunity compared with younger people and the increasing frequency of non-communicable comorbidities associated with age.28 44 45 Underlying conditions and comorbidities, such as hypertension, diabetes and cardiovascular disease, have been found to further exacerbate COVID-19 disease progression and fatality.46 47 Chronic diseases and comorbidities may themselves be gendered, generated through gendered behavioural and social pressures or expectations.48 While our study supports global trends during this time period,3 25 49 further research is necessary and encouraged to understand the interaction between sex and age, particularly in an SSA context. In people less than 40 years, we found no differences in CFR among sexes as confirmed in European countries,50 51 Peru,52 Canada, China and Korea53 in a similar period of time. In contrast, a large study in China highlighted a significant sex-specific difference in CFR in patients aged 30 years or older.54 Further studies need to confirm our findings. However, based on our results, prevention activities should target men and women over the age of 40 years.
Strengths and limitations of the study
Thus far, to the best of our knowledge, this is one of the largest studies exploring sex-specific differences in CFRs at regional and country level of SSA. Our data were derived from individual-level patient data, which included information on age and sex, for a consecutive duration of 120 days from 20 SSA countries. Due to data unavailability, we were only able to include 20 from the 47 member states comprising the WHO African region. A particular strength of our study included our choice of prior. Our sensitivity analysis indicated that our prior was robust, since the results from all priors considered produced similar results. Among the 20 countries, only four countries had low sample sizes in some age categories, suggesting a strong statistical validity and accuracy among the remaining 16 countries. Our study, however, goes not without limitations. We excluded 12 851 cases with missing information about the variables of interest, but this could lead to some biases in the results. Additionally, our dataset was solely limited to age and sex; other gender-relevant indicators, such as presence of comorbidities, other health risk factors (as cholesterol levels and oestrogen levels), prevalence of diseases such as HIV and TB and socioeconomic status, were not available for analysis, preventing us from stratifying and conducting further analyses on gender-related health inequities. Furthermore, gendered hospital admissions and differential treatment for men and women in clinical settings could act as a confounding source of bias to COVID-19 case and death reporting, impacting our CFR results. Also, the lack of diagnostic capacity and under-reporting of deaths during the early stage of the pandemic could have affected our results and could also have a gender bias. Due to lack of data, we could not address this. Moreover, case definition, surveillance capacity (eg, variation in testing rates) and data management varied from country to country, which made comparisons across countries complicated. Besides, given that we did not include all the SSA countries, our results may not be generalisable to the whole region.
Conclusions
Our results suggest that both sex and age are important predictors of COVID-19 mortality in Africa, as in other regions. Sex-disaggregated data and analyses are imperative to identity target risk groups and thus reduce differences in COVID-19 exposure and vulnerability for both women and men in SSA.55 In addition to the collection of sex-disaggregated data on COVID-19 prevalence and mortality, disaggregated data on testing rates, hospitalisations and healthcare workers provide further insights into COVID-19 sex differences and should not be overlooked in future studies. Our study points out the need for further research on how HIV and noncommunicable diseases (NCDs) may have affected the progression of COVID-19 infection and mortality among women and men in SSA. Although not analysed in our study, it is important to emphasise that age and sex do not act alone but intersect with other social determinants of health to influence COVID-19 disease progression and mortality. Since gender-responsive policies were implemented only in five African countries (only two of them included in our analysis) and in few other countries all over the world,15 our findings raise awareness on the importance of taking into account sex and gender in developing public health responses. In order to design and promote gender-sensitive public health interventions, it is essential that SSA countries collect and report up-to-date COVID-19 statistics that are disaggregated by sex and age and other social categories, such as class, economic status and ethnicity.16
Data availability statement
Data are available on reasonable request. The data (data dictionary, statistical code) that support the findings of this study are available upon reasonable request from the corresponding author. Some of the data are publicly available through situation reports produced by Ministries of Health and WHO/AFRO on their respective websites.
Ethics statements
Patient consent for publication
Acknowledgments
We would like to express our sincere appreciation and gratitude to the entire team at the GRAPH Network and the WHO Regional Office for Africa for guiding and supporting us along the entirety of the study. Additionally, we would like to thank the governments of Angola, Botswana, Burkina Faso, Chad, Congo, Eswatini, Gambia, Guinea, Kenya, Liberia, Mauritius, Mozambique, Namibia, Niger, Rwanda, São Tomé e Principe, Sierra Leone, Senegal, Seychelles and Uganda for their contribution to the merge line listing dataset.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
JD and IT are joint first authors.
IT, OK and FCC are joint senior authors.
Handling editor Seye Abimbola
Twitter @AnanthuJames
JD, IT, OK, OK, FCC and FCC contributed equally.
Contributors JD and IT conceived the study with the support of GGD, AJ, BN and AV and with the supervision of OK and FCC. JD and BN acquired, cleaned and analysed the data. JD performed the statistical analysis with the supervision and support of FCC and AJ. JD, AJ and IT interpreted the data. JD, IT, AJ, GGD, BN, AV, SBM and and LNTR participated in weekly discussions that were helpful in exchanging ideas and making progress. IT, GGD and LNTR conducted the literature search. JD, IT, AJ, GGD, AV and BN drafted the initial manuscript, which was improved by JD, IT, GGD, AJ and later finalised by JD, IT and AJ. FCC, BI, FM, GT, OK, CS and JLA critically revised the manuscript for important intellectual content and supervised the study. All authors contributed to final approval of the version to be submitted. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. JD, IT, FCC and OK are the guarantors.
Funding OK was funded by the Swiss National Science Foundation (grants no. 196270 and 163878).
Map disclaimer The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.