-
PDF
- Split View
-
Views
-
Cite
Cite
P. Astolfi, L.A. Zonta, Risks of preterm delivery and association with maternal age, birth order, and fetal gender, Human Reproduction, Volume 14, Issue 11, November 1999, Pages 2891–2894, https://doi.org/10.1093/humrep/14.11.2891
Close - Share Icon Share
Abstract
The risk of preterm delivery in a recent sample (1990–1994) of Italian liveborns was examined, taking into account child birth order, and maternal age and education in addition to the fetal gender. Univariate analyses showed that a higher risk was associated with male than female babies, with first- than second-born children, with older mothers, and with less educated mothers. The relative weights of the factors examined were evaluated through logistic regression analyses and the highest and the lowest risks were found to be associated with advanced maternal age and male fetal gender respectively. Our findings therefore suggest that biological factors associated more with advanced maternal age than with the male gender of the fetus may influence premature onset of labour.
Introduction
Gestational age is the second best prognostic factor, after baby weight, for the outcome of pregnancy. Apart from pathological conditions, several factors associated with a preterm delivery have been extensively investigated since the 1950s (Karn and Penrose, 1951; Wilcox and Skjoerven, 1992). On average, a lower gestational age is observed among male compared with female babies. It has been suggested that a preterm delivery may be induced by fetal gender (Cooperstock and Campbell, 1996). These workers reported an excess of males among preterm babies, in agreement with previous findings (Hall and Carr-Hill, 1982; McGregor et al., 1992), suggesting that male fetal gender, hormonally involved in the control of labour onset, might be responsible for the shortened duration of pregnancy. Possible determinants of the variation in the male to female ratio with pregnancy duration have been extensively discussed. In particular, a U-shaped variation has been suggested, which could be related to time of fertilization in the cycle (James, 1994, 1997a; Bernstein, 1998a, 1998b).
In order to investigate the importance of fetal sex in comparison with other factors, we examined the risk of preterm delivery in a recent sample of Italian births. In addition to fetal gender we included in the analysis three other characteristics well known to influence not only neonatal survival and health, but also pregnancy duration: maternal age at delivery, maternal educational level, and the baby birth order.
Maternal age, which has been widely reported to influence pregnancy outcome (Fretts et al., 1995; Lansac, 1995; Breart, 1997; Zonta et al., 1997; Astolfi et al., 1999) and which therefore might also affect pregnancy duration, is increasing in importance since the frequency of mothers bearing children at advanced age has been rising over the last 20 years. Maternal educational level, which is generally accepted as a reliable indicator of the socio-economic status of the family, might at least partially account for variation in gestational age, because of the differences in availability of antenatal care.
Babies of different birth orders, who have been shown to run different stillbirth risks (Astolfi et al., 2000), might also be differently exposed to the risk of a shortened pregnancy. At present, however, because of the reduced size of Italian families, a comparative analysis is suitable only between first- and second-borns. The analysis of only the first two orders has therefore avoided the problem of possible interactions between fetal gender and maternal age discussed by several authors in relation to deliveries of very high order (Almagor et al., 1998; James, 1998).
Materials and methods
Subject population and variables
Data were obtained on the authors' special request from the Italian National Institute of Statistics (ISTAT, Rome, Italy) and consisted of 2 796 334 birth records from 1990 to 1994. In order to deal with a homogeneous sample and to eliminate the confounding effects of some factors, such as stillbirth, twinning and unfavourable familial situations, from the original sample, we selected 2 300 127 children, who were legitimate liveborns of first (1 344 195) or second (955 932) birth order. In fact, because of the reduced size of Italian families (total fertility rate was as low as 1.2 children per woman in 1995) the first two birth orders covered over 80% of total births.
For each newborn, information was available on gender (SX), birth order (BO), gestational age (GA), maternal age (MA), and educational level (ED). Gestational age, estimated from last menstrual date, ranged from 20 to 44 weeks, and deliveries occurring before the 37th week were considered preterm, in accordance with the World Health Organization (WHO) definition (http://www.who.int/p11/ter/). The risk of preterm delivery was evaluated in mothers older than 30 years, grouped into three age classes (30–34, 35–39, ⩾40), which covered the upper 50% in the maternal age distribution. As for maternal education, the best available indicator of the family socio-economic level, two levels (>8 and ⩽8 years of schooling) were considered.
Data analysis
Univariate analyses were carried out to evaluate the effects of single factors on pregnancy duration and the risk of preterm delivery.
The relative risk of preterm delivery was estimated by logistic regression analyses, with fetal gender and birth order, the maternal age and education, and interactions of all factors included as explanatory variables.
Statistical analyses were performed by means of SPSS/PC 8.0 and SAS V6.10 procedures.
Results
Univariate analyses were first performed to evaluate the influence of some characteristics of the baby (birth order and gender) and of the mother (age and education) on the risk of a preterm delivery.
Birth order
In agreement with previous and current observations that second children are generally less prone to the risk of stillbirth (Ulizzi et al., 1998; Astolfi et al., 2000), a significantly lower percentage of preterm babies was found among second- compared with first-borns (3.894% versus 4.426%; odds ratio (OR) = 0.875, 95% confidence interval (CI) 0.863–0.887).
Maternal educational level
Antenatal care, which may be differently available according to family socio-economic and cultural levels, may have influenced not only the outcome but also the duration of the pregnancy. We therefore evaluated the risk of preterm delivery with respect to maternal education. As expected, among the less educated mothers a significantly higher quota of preterm deliveries occurred (4.4 versus 4.0%, OR 1.09 95% CI 1.076–1.105), and the risk was not significantly different between first and second deliveries.
Maternal age
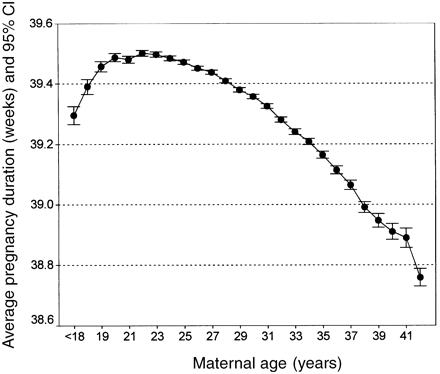
Mean pregnancy duration behaved as a second-degree function as maternal age increased: it reached the maximum length (39.5 weeks) in 22-year-old mothers and the absolute minimum (about 38.75 weeks) in mothers older than 41 years (Figure 1). However, the difference between the extreme values, which amounted to less than 1 week, seemed negligible from the biological point of view.
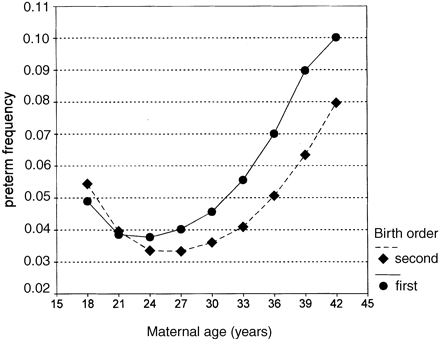
In contrast, when the frequency of preterm deliveries was considered, maternal age represented an important risk factor: preterm deliveries occurred in well below 4% of births among mothers younger than 35 years, which was almost half the frequency observed among the older mothers (⩾35 years). This difference was particularly striking among the first children born to mothers over 35 years (Figure 2). When the same graph was plotted but separating male from female babies, no effect of maternal age over fetal gender was found (data not shown). In other words, the effect of maternal age was consistent over fetal gender.
Baby gender
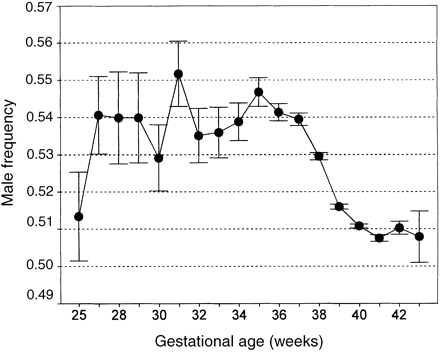
Male babies are known to have a significantly higher risk of being preterm than female babies (4.4 versus 4.0%, OR 1.109, 95% CI 1.095–1.124). In agreement with previous reports (Hall and Carr-Hill, 1982; McGregor et al., 1992; Cooperstock and Campbell, 1996), we also observed an excess of males among preterm compared to term babies, the male proportion being 0.541 and 0.515 respectively. No significant difference was found between first and second children. The week by week variation in the proportion of males among preterm babies showed a pattern very similar to that recently reported (Cooperstock and Campbell, 1996). Except for the low value observed among extremely premature babies (20–25 weeks), the male proportion was consistently high up to the 37th week, after which a sharp decrease occurred (Figure 3).
Since a significant though small relationship was revealed in the monofactorial analyses between the four factors and the risk of preterm delivery, an estimate of their relative weight by means of multifactorial analyses was obtained.
Multifactorial analyses
The risk of preterm birth was evaluated by logistic regression as a function of the gender and birth order of the baby, the maternal age (from 30 years onwards), the number of years of schooling, and their interactions. Since no order interactions were found to be significant, the simplest model with only the main factors was considered. According to this model, first-born children had a higher risk than second-borns, and maternal age seemed to be the most important factor contributing to an increased risk of premature babies (Table I). The relative weights of low educational level and male gender were lower but still significant. From the total sample equation, the predicted minimum and maximum probabilities of a preterm birth, computed considering all favourable and all unfavourable conditions respectively, turned out to be Pmin = 0.033 and Pmax = 0.118. This was interpreted as meaning that a first-born male child delivered to an uneducated mother older than 39 years had a risk of being born preterm of more than 3.5 times higher than a second-born female child delivered to a well-educated mother aged 30–34 years.
The significantly increased risk run by first-born children in comparison with second-borns suggested that independent logistic regression analyses should be carried out on the two sub-samples separately. The results of these analyses confirmed that whatever the child birth order, maternal age accounted for the highest weight while baby gender accounted for the lowest (Table I).
Discussion
Though all the characteristics of the mother and the baby included in the analyses were significant risk factors for preterm delivery, maternal age accounted for the highest weight, as shown by the results of logistic analyses (Table I). The frequency of preterm deliveries was lowest in mothers aged between 20 and 30 years, but this increased sharply as maternal age increased (Figure 2). Contrasting the mothers <35 and ⩾35 years, which is generally considered to be the threshold age for baby and maternal health risk, the respective preterm percentages were 4.0 and 6.37 (OR 1.635, 95% CI 1.604–1.666).
The high quota of preterm births in very young (<20 years) mothers (Figure 2) may be indicative of unfavourable socio-economic situations: the current average maternal age at delivery is approaching 30 years (Ulizzi et al., 1998) and only 3.2% of mothers face childbirth at a very young age. In addition, it cannot be excluded that the weeks of gestation may be underestimated more frequently in poorly educated and/or in very young mothers (James, 1997b).
Baby birth order, maternal education, and fetal gender were of descending importance though always significant. Second-born children, previously reported to be the least prone to stillbirth risk (Ulizzi et al., 1998), seem to be less vulnerable even to preterm risk; and in spite of the improvement in health care, less educated mothers still run a higher risk of preterm deliveries, probably due to the relative lack of prenatal care in unfavourable living conditions. Finally, the factor with the lowest weight in assessing a preterm delivery risk was found to be fetal gender.
In conclusion, in our sample we found a clear indication that advanced age of the mother, more than male gender of the baby, contributes an increased risk of preterm delivery. Biological, most probably hormonal, factors related to maternal ageing seem more important than the sex of the fetus in determining the premature onset of labour.
On the other hand, the excess of males among preterm babies is in agreement with the general finding of biological and even genetic weakness of male fetuses, witnessed by the high sex ratio observed in unfavourable pregnancy outcome. In fact a higher male proportion holds not only for stillborns, but also for spontaneously aborted fetuses (Cann and Cavalli-Sforza, 1968; Hassold et al., 1983; Jakobovits, 1991; Zonta et al., 1996). It should be noted, however, that appropriate health care might increase the number of preterm babies born alive even after a very short gestation, i.e. those babies who otherwise would have been either aborted or stillborn.
Logistic regression equations for the probability (P) of a preterm birth according to the simplest models that fitted the data well. Odds ratios and 95% confidence intervals (in parentheses) for each factor are shown
| Factor . | Total sample . | First-borns . | Second-borns . |
|---|---|---|---|
| Total sample: logit (p) = –4.400 + 0.360 MA + 0.102 SX + 0.232 ED + 0.321 BO. | |||
| First-borns: logit (p) = –3.737 + 0.375 MA + 0.078 SX + 0.228 ED. | |||
| Second-borns: logit (p) = –4.098 + 0.346 MA + 0.124 SX + 0.234 ED. | |||
| Birth order (BO) | 1.3788 | — | — |
| (1.3508–1.4073) | — | — | |
| Maternal age (MA) | 1.4328 | 1.4550 | 1.4139 |
| (1.4081–1.4580) | (1.4185–1.4925) | (1.3806–1.4481) | |
| Education (ED) | 1.2608 | 1.2562 | 1.2636 |
| (1.2352–1.2869) | (1.2196–1.2940) | (1.2282–1.2999) | |
| Gender (SX) | 1.1070 | 1.0811 | 1.1322 |
| (1.0847–1.1298) | (1.0499–1.1131) | (1.1004–1.1648) | |
| Factor . | Total sample . | First-borns . | Second-borns . |
|---|---|---|---|
| Total sample: logit (p) = –4.400 + 0.360 MA + 0.102 SX + 0.232 ED + 0.321 BO. | |||
| First-borns: logit (p) = –3.737 + 0.375 MA + 0.078 SX + 0.228 ED. | |||
| Second-borns: logit (p) = –4.098 + 0.346 MA + 0.124 SX + 0.234 ED. | |||
| Birth order (BO) | 1.3788 | — | — |
| (1.3508–1.4073) | — | — | |
| Maternal age (MA) | 1.4328 | 1.4550 | 1.4139 |
| (1.4081–1.4580) | (1.4185–1.4925) | (1.3806–1.4481) | |
| Education (ED) | 1.2608 | 1.2562 | 1.2636 |
| (1.2352–1.2869) | (1.2196–1.2940) | (1.2282–1.2999) | |
| Gender (SX) | 1.1070 | 1.0811 | 1.1322 |
| (1.0847–1.1298) | (1.0499–1.1131) | (1.1004–1.1648) | |
Logistic regression equations for the probability (P) of a preterm birth according to the simplest models that fitted the data well. Odds ratios and 95% confidence intervals (in parentheses) for each factor are shown
| Factor . | Total sample . | First-borns . | Second-borns . |
|---|---|---|---|
| Total sample: logit (p) = –4.400 + 0.360 MA + 0.102 SX + 0.232 ED + 0.321 BO. | |||
| First-borns: logit (p) = –3.737 + 0.375 MA + 0.078 SX + 0.228 ED. | |||
| Second-borns: logit (p) = –4.098 + 0.346 MA + 0.124 SX + 0.234 ED. | |||
| Birth order (BO) | 1.3788 | — | — |
| (1.3508–1.4073) | — | — | |
| Maternal age (MA) | 1.4328 | 1.4550 | 1.4139 |
| (1.4081–1.4580) | (1.4185–1.4925) | (1.3806–1.4481) | |
| Education (ED) | 1.2608 | 1.2562 | 1.2636 |
| (1.2352–1.2869) | (1.2196–1.2940) | (1.2282–1.2999) | |
| Gender (SX) | 1.1070 | 1.0811 | 1.1322 |
| (1.0847–1.1298) | (1.0499–1.1131) | (1.1004–1.1648) | |
| Factor . | Total sample . | First-borns . | Second-borns . |
|---|---|---|---|
| Total sample: logit (p) = –4.400 + 0.360 MA + 0.102 SX + 0.232 ED + 0.321 BO. | |||
| First-borns: logit (p) = –3.737 + 0.375 MA + 0.078 SX + 0.228 ED. | |||
| Second-borns: logit (p) = –4.098 + 0.346 MA + 0.124 SX + 0.234 ED. | |||
| Birth order (BO) | 1.3788 | — | — |
| (1.3508–1.4073) | — | — | |
| Maternal age (MA) | 1.4328 | 1.4550 | 1.4139 |
| (1.4081–1.4580) | (1.4185–1.4925) | (1.3806–1.4481) | |
| Education (ED) | 1.2608 | 1.2562 | 1.2636 |
| (1.2352–1.2869) | (1.2196–1.2940) | (1.2282–1.2999) | |
| Gender (SX) | 1.1070 | 1.0811 | 1.1322 |
| (1.0847–1.1298) | (1.0499–1.1131) | (1.1004–1.1648) | |
The average pregnancy duration in weeks and its 95% confidence interval (CI) plotted against maternal age in years. The last point refers to mothers aged over 41 years.
Preterm delivery frequency plotted against maternal age, grouped into 3-year intervals, except for classes 18 and 42, which respectively comprised all mothers under 20 and over 40 years. The two curves refer to first- (•) and second- (⧫) born children.
Frequency of males (±SD) plotted against pregnancy duration (weeks). The two extreme points show the values computed in babies with a gestational age of 20–26 weeks (lower) and of >42 weeks (higher).
To whom correspondence should be addressed
This study was financed by the Ministero della Università e della Ricerca Scientifica and by the University of Pavia. We wish to thank Mrs S. Zanoli for technical help.
References
Almagor, M., Schwed, P. and Yaffe H. (
Astolfi, P., Ulizzi L. and Zonta, L.A. (
Astolfi, P., Ulizzi L. and Zonta L.A. (2000) Natural selection and reproductive behavior. Hum. Biol. (in press).
Bernstein, M.E. (
Cann, H.M. and Cavalli-Sforza, L.L. (
Cooperstock, M. and Campbell, J. (
Fretts, R.C., Schmittdiel, J., McLean, F.H. et al. (
Hall, M.H. and Carr-Hill, R. (
Hassold, T., Quillen, S.D. and Yamane, J.A. (1983) Sex ratio in spontaneous abortions. Ann. Hum. Genet.47, 39–47.
Jakobovits, A.A. (
James, W.H. (
James, W.H. (
James, W.H. (
Karn, M.N. and Penrose, L.S. (
Lansac, J. (
McGregor, J.A., Leff, M., Orleans, M. and Baron, A. (
Ulizzi, L., Astolfi, P. and Zonta, L.A. (
World Health Organization (WHO) http://www.who.int/p11/ter/
Wilcox, A.J. and Skjoerven, R. (
Zonta, L.A., Astolfi, P. and Vlizzi, L. (