Article Text
Abstract
Introduction As new vaccines are developed more vaccine coadministrations vaccines are being offered to make delivery more practical for health systems and patients. We compared the safety of coadministered vaccines with separate vaccination for 20 coadministrations by considering nine types of adverse events following immunisation (AEFI).
Methods Real-life immunisation and adverse event data for this observational cohort study were extracted from the Oxford-Royal College of General Practitioners Research and Surveillance Centre for children registered in the database between 2008 and 2018. We applied the self-controlled case series method to calculate relative incidence ratios (RIR) for AEFI. These RIRs compare the RI of AEFI following coadministration with the RI following separate administration of the same vaccines.
Results We assessed 3 518 047 adverse events and included 5 993 290 vaccine doses given to 958 591 children. 17% of AEFI occurred less and 11% more frequently following coadministration than would have been expected based on the RIs following separate vaccinations, while there was no significant difference for 72% of AEFI. We found amplifying interaction effects for AEFI after five coadministrations comprising three vaccines: for fever (RIR 1.93 (95% CI 1.63 to 2.29)), rash (RIR 1.49 (95% CI 1.29 to 1.74)), gastrointestinal events (RIR 1.31 (95% CI 1.14 to 1.49)) and respiratory events (RIR 1.27 (1.17–1.38)) following DTaP/IPV/Hib+MenC+ PCV; gastrointestinal events (RIR 1.65 (95% CI 1.35 to 2.02)) following DTaP/IPV/Hib+MenC+ RV; fever (RIR 1.44 (95% CI 1.09 to 1.90)) and respiratory events (RIR 1.40 (95% CI 1.25 to 1.57)) following DTaP/IPV/Hib+PCV+ RV; gastrointestinal (RIR 1.48 (95% CI 1.20 to 1.82)) and respiratory events (RIR 1.43 (95% CI 1.26 to 1.63)) following MMR+Hib/MenC+PCV; gastrointestinal events (RIR 1.68 (95% CI 1.07 to 2.64)) and general symptoms (RIR 11.83 (95% CI 1.28 to 109.01)) following MMR+MenC+PCV. Coadministration of MMR+PCV led to more fever (RIR 1.91 (95% CI 1.83 to 1.99)), neurological events (RIR 2.04 (95% CI 1.67 to 2.49)) and rash (RIR 1.06 (95% CI 1.01 to 1.11)) compared with separate administration, DTaP/IPV/Hib+MMR to more musculoskeletal events (RIR 3.56 (95% CI 1.21 to 10.50)) and MMR+MenC to more fever (RIR 1.58 (95% CI 1.37 to 1.82)). There was no indication that unscheduled coadministrations are less safe than scheduled coadministrations.
Conclusion Real-life RIRs of AEFI justify coadministering routine childhood vaccines according to the immunisation schedule. Further research into the severity of AEFI following coadministration is required for a complete understanding of the burden of these AEFI.
- vaccines
- immunisation
- prevention strategies
- public health
Data availability statement
Data may be obtained from a third party and are not publicly available. Data used for this study remains stored on secure servers of the Oxford-Royal College of General Practitioners (RCGP) Research and Surveillance Centre (RSC), and can be accessed on the RCGP RSC conditions.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
Vaccine coadministration may lead to interactions between individual products and alter health outcomes. Information about the safety of real-life vaccine coadministrations versus separate vaccinations is scarce and a potential source for vaccine hesitancy.
WHAT THIS STUDY ADDS
Coadministering two vaccines decreases the relative incidence of severaladverse events following immunisation (AEFI) compared with separately administering the respective vaccines, while adding a third vaccine can lead to a higher than expected relative incidence of AEFI.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Real-life relative incidence ratios of AEFI justify the coadministration of routine childhood vaccines as recommended in immunisation schedules. Nevertheless, health systems should run enhanced surveillance for a comprehensive monitoring of the burden of AEFI following vaccine coadministration.
Introduction
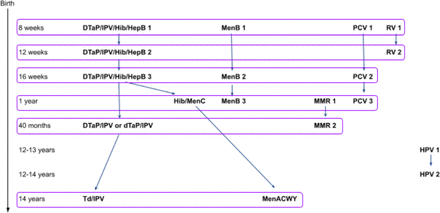
As new vaccines are developed to protect against a growing number of vaccine-preventable diseases, vaccine coadministrations will gain importance to make immunising more practicable for health systems and patients globally. Vaccine coadministration practices cost-effectively facilitate the introduction of new vaccines into immunisation programmes and improve coverage rates.1–5 According to the National Health Service and Public Health England’s immunisation schedule for 2018, between two and four vaccines were scheduled for coadministration at six time points between birth and 14 years, adding up to 17 vaccines (first and subsequent doses) for 16 different antigens (figure 1).6 However, coadministering vaccines may lead to interactions between individual products and alter their health outcomes.7–9 Therefore, insights in the effectiveness and safety profiles of vaccine coadministration are essential to inform vaccination regimens.9 Furthermore, safety information can overcome uncertainties about the health outcomes of coadministered vaccines, which is a driver for vaccine hesitancy in parents.10 11
Coadministrations in the routine paediatric immunisation schedule NHS 2018.6 NHS, National Health Service.
All recommended paediatric routine immunisations can be coadministered and there are no recommendations against coadministration, unless reported in the Summary of Product Characteristics.12 13 Coadministration is explicitly endorsed by the WHO for some vaccines, while it does not mean that the vaccines without such endorsement cannot be coadministered.14 Furthermore, studying the safety of paediatric immunisation schedules, for example, whether health outcomes differ for children who receive fewer immunisations per physician visit, is recommended by the Institute of Medicine.15 A recent literature review showed that the safety of vaccine coadministrations versus separate vaccinations is mostly assessed in prelicensure clinical trials, while data on the extent and impact of vaccine coadministrations in real life postlicensure are scarce.16 To fill this gap, we compared the safety of coadministering vaccines versus the safety of separately administering the same vaccines for 20 coadministrations including real life both schedule and off-schedule coadministrations
Methods
The study population and data collection methods were previously described in detail.17 18 In brief, data for our observational cohort study were extracted from the Oxford-Royal College of General Practitioners Research and Surveillance Centre, a national, electronic primary healthcare medical record database, representative of the English population.19 20 We included all children between 0 and 18 years old during the study period from 1 January 2008 to 31 December 2018. Children were excluded from analyses if they were registered in the database after the scheduled age for the first dose of a vaccine. The extracted data were pseudonymised and managed according to privacy and data protection regulations. Neither patients nor the public were involved in this study.
We included paediatric vaccines that were given in the 10 most frequent vaccine coadministrations according to the immunisation schedule and the ten most frequent unscheduled coadministrations (vaccines that were never scheduled together) between 2008 and 2018: DTaP/IPV/Hib, DTaP/IPV, dTaP/IPV, Td/IPV, MMR, PCV, MenB, MenC, Hib/MenC, RV and HPV.6 18 21–28 The selected vaccine coadministrations are presented in table 1.18 An overview of the changes in the immunisation schedule during the study period has been documented before.17 We collected the vaccination types and dates for each vaccination. Records with a missing patient-ID, vaccination type or date were excluded. We selected 33 potential adverse events following immunisation (AEFI) based on their occurrence in previous studies16 and grouped these in 9 types of AEFI as listed in table 2. All event dates during the study period for each of the included children were collected.
Number of scheduled and off-schedule vaccine coadministrations18
Frequency of adverse events included in the study
We used the self-controlled case series (SCCS) method to compare the relative incidences (RI) of each type of AEFI after vaccine coadministration with their RI after separate administrations of the same vaccines. The RI compares the incidence of events in a risk period with the incidence in a control period for the same individual. The risk period was defined as 42 days postvaccination. Events in overlapping risk periods were allocated to the most recent exposure. The unexposed period encompassed the remaining time that children were registered in the database during the study period while between 0 and 18 years of age, whereby the observation period was partitioned by ages.
The SCCS model estimates the RI of an AEFI for each vaccine in absence of other vaccines, corresponding to a separate vaccine administration. These RIs are estimated by a fitted SCCS conditional Poisson model using the SCCS method.29 30 When estimating the RI as a dependent variable, the regression model includes the independent variables: age effects; exposure effects of each of the separate vaccines; exposure effects of any vaccines coadministered. The latter covariate is thus an interaction term for the effect of coadministration on the individual vaccines’ RIs. This term can be interpreted as an RI ratio (RIR) (RIRinteraction) because it corresponds to the ratio of the RI in the coadministration group (RIcoadministered) compared with the RI in the designated reference group with separate vaccinations (eg, RIvaccine a, RIvaccine b).31 The factors relate as follows:
RIRinteraction = RIcoadministered / (RIvaccine a x RIvaccine b)
An interaction term significantly less than 1 (p<0.05) indicates an inhibitory interaction effect as the RIcoadministered will be lower than expected based on the RIs of the separately administered vaccines. An interaction term significantly greater than 1 (p<0.05) indicates an amplifying interaction effect. Vaccination ages were included as a vector in the SCCS model to stratify the analyses and account for age-related differences in incidences. These analyses were performed in R32 using the SCCS package.33
Results
A total of 5 993 290 vaccine doses delivering 13 920 730 antigen exposures to 958 591 children met our inclusion criteria for analysis. This study population was representative for the entire population in the database.17 Twenty per cent of the included vaccines were given separately, while 80% were coadministered: 37% were coadministrations of two, 34% were coadministrations of three and 8% were c-administrations of four vaccines. The patterns of coadministration for each vaccine are shown in figure 2. Our study included 3 518 047 adverse events, which are categorised and quantified in table 2. The numbers of adverse events in the control and risk periods, which were included in the SCCS analysis, are listed in table 3.
Proportions of routine paediatric vaccines coadministered.
Numbers of adverse events in the risk and control periods, included in the self-controlled case series analysis
Coadministrations of two vaccines
Table 4 presents the RIRs of the adverse events analysed following vaccine coadministrations. The RIs of adverse events following coadministration of DTaP/IPV/Hib+PCV, DTaP/IPV or dTaP/IPV+Hib/MenC, DTaP/IPV or dTaP/IPV+MMR, DTaP/IPV or dTaP/IPV+PCV, MMR+Td/IPV or Td/IPV+HPV were not increased as compared with the separate administration of these vaccines. The RIs of respirato–ry events were lower (RIR≤1, p<0.05) than expected based on the separate immunisations after all coadministrations of two vaccines except Td/IPV+HPV. We also found lower RIs of gastrointestinal events after seven, and less local events and rash after each three coadministrations of two vaccines.
(A) Relative incidence ratios (RIR) and interaction effects of the adverse events for all recommended coadministrations studied. (B) RIR and interaction effects of the adverse events for all never recommended coadministrations studied
While the coadministration of MMR+PCV had an inhibitory interaction effect on gastrointestinal events, local symptoms and respiratory events, it led to a higher RI of fever (RIR 1.91, 95% CI 1.83 to 1.99), neurological events (RIR 2.04, 95% CI 1.67 to 2.49)—particularly convulsions—and rash (RIR 1.06, 95% CI 1.01 to 1.11). Also coadministration of DTaP/IPV/Hib+MMR led to a higher RI of musculoskeletal events (RIR 3.56, 95% CI 1.21 to 10.50) and MMR+MenC to a higher RI of fever (RIR 1.58, 95% CI 1.37 to 1.82).
Fever and neurological events occurred less frequently (RI<1) after the vaccination of either separate or coadministration of DTaP/IPV/Hib+MenC, compared with the control periods. We observed the same for fever following DTaP/IPV/Hib+RV. However, the RIRs of these AEFI after coadministration indicated an amplifying interaction effect compared with separate vaccinations (RIR>1, p<0.05), although this effect did not raise the resulting RI’s following coadministration above 1. Thus, these AEFIs remained less frequent than in the control periods.
Coadministrations of three vaccines
While the coadministration of DTaP/IPV/Hib+PCV had an inhibitory interaction effect on fever, gastrointestinal events, rash and respiratory events compared with these vaccines’ separate administrations, adding a third vaccine was associated with an RIR>1 (p<0.05) for these events in the coadministration of, DTaP/IPV/Hib+MenC + PCV (RIR 1.93, 95% CI 1.63 to 2.29; RIR 1.31, 95% CI 1.14 to 1.49; RIR 1.49, 95% CI 1.29 to 1.74; RIR 1.27, 95% CI 1.17 to 1.38). As a result, the RIs of these AEFI were higher than what would have been expected based on the RIs of these vaccines’ separate administrations—particularly for diarrhoea, acute conjunctivitis and cough. Similarly, despite the inhibitory effect on gastrointestinal and respiratory events of DTaP/IPV/Hib+PCV, DTaP/IPV/Hib+MenC and DTaP/IPV/Hib+RV, the RI of gastrointestinal events—particularly vomiting—was higher after DTaP/IPV/Hib+MenC+RV (RIR 1.65, 95% CI 1.35 to 2.02) and the RI of respiratory events—articularly acute conjunctivitis, cough and wheezing—was higher after DTaP/IPV/Hib+PCV+RV (RIR 1.40, 95% CI 1.25 to 1.57). The latter also resulted in more fever (RIR 1.44; 95% CI 1.09 to 1.90). For the other AEFI included in this study, there was an inhibitory or no significant effect on the RIs following coadministration of DTaP/IPV/Hib+MenB+PCV, DTaP/IPV/Hib+MenC+PCV, DTaP/IPV/Hib+MenC+RV and DTaP/IPV/Hib+PCV+RV (see table 4).
Coadministering MMR+MenC and MMR+PCV had an inhibitory interaction effect on gastrointestinal and respiratory events, as well as local symptoms (erythema) for the latter, compared with separate vaccine administrations, while coadministering MMR+MenC+PCV was associated with an RIR>1 (p<0.05) for gastrointestinal events (RIR 1.68, 95% CI 1.07 to 2.64)—particularly vomiting—and general symptoms (RIR 11.83, 95% CI 1.28 to 109.01). Also the RIRs for gastrointestinal (RIR 1.48, 95% CI 1.20 to 1.82)—particularly diarrhoea and vomiting—and respiratory events (RIR 1.43, 95% CI 1.26 to 1.63)—acute conjunctivitis and cough—were >1 (p<0.05) after MMR+Hib/MenC+PCV. There was no or an inhibitory interaction effect of coadministering MMR+Hib/MenC+PCV, MMR+MenC + PCV, or DTaP/IPV or dTaPIPV+MMR+Hib/MenC on the other events included in this study (see table 4).
Coadministration of four vaccines
Adding a fourth vaccine did not significantly alter the amplifying effects observed when coadministering three vaccines for any of the investigated AEFI.
Discussion
The RIs following vaccine coadministration for most of the analysed AEFI (72%) were not significantly different from what would have been expected based on the RIs following separate administration of the respective vaccines, while we found an amplifying effect following coadministration for 11% and an inhibitory effect for 17% of AEFI studied. Although studies comparing the safety of coadministration with separate vaccination are rare, an earlier literature review found increased AEFI following coadministration in 16% of studies, less AEFI following coadministration in 10% of studies, while the majority of studies found no statistically significant differences in the incidence of any AEFI following coadministration compared with separate administration of the same vaccines.16 We found more differences in the incidence between coadministration and separate administration of vaccines, likely because our study was designed specifically to detect such differences while the majority of reviewed studies were clinical trials not designed to demonstrate statistically significant safety differences.16
Half of the 20 investigated vaccine coadministrations led to a higher reactogenicity for at least one AEFI. We found amplifying interaction effects for five out of seven investigated coadministrations of three vaccines. Such an increased reactogenicity is often reported when coadministering three vaccines. DTaP/IPV/Hib+MenC+PCV led to more fever, rash, gastrointestinal and respiratory events compared with the separate administration of these vaccines. Other studies also reported fever, local and general symptoms, and gastrointestinal events following this coadministration.34 35 We found increased gastrointestinal events (vomiting) after DTaP/IPV/Hib+MenC+RV compared with separate administration, which were also detected in another study, together with general symptoms.16 36 DTaP/IPV/Hib+PCV+RV led to more fever and respiratory events compared with separate administration. Fever, local and general symptoms, and gastrointestinal events were often reported in another study on DTaP/IPV/Hib+PCV+RV coadministration.37 Also studies on DTaP/IPV/Hib/HepB+PCV+RV reported mostly fever, local reactions, respiratory and gastrointestinal events.38 39 MMR+Hib/MenC+PCV led to more gastrointestinal, and respiratory events and less fever and musculoskeletal events than would have been expected based on separate vaccinations. One clinical trial on this coadministration did not detect differences for local or systemic adverse events compared with separate administrations.40 One of the unscheduled coadministrations of three vaccines—MMR+MenC+PCV—led to more than expected gastrointestinal events and general symptoms and less fever. No other studies investigated the safety of the unscheduled coadministrations of three vaccines. One scheduled coadministration of two vaccines—MMR+PCV led to more fever, neurological events, and rash compared with separate administration. One other study reported lower40 and another one higher proportions41 of fever, while the other AEFIs were not specifically assessed or reported in these and other studies on MMR+PCV.16 40–42 Also the unscheduled coadministrations of DTaP/IPV/Hib+MMR caused more musculoskeletal events and MMR+MenC more fever than expected. One study reported an increase in overall AE following DTaP/IPV/Hib+MMR16 43 and another detected increased AE following coadministrations of MMR+MenC, particularly febrile seizures.44
For coadministrations of two vaccines, we detected amplifying interaction effects for events that had an RI<1 following vaccination and thus occurred less following immunisation than in the control period. Although the RIs of these events were higher following coadministration than would have been expected based on separate administration of these vaccines, they still occurred less than in the control period (RI<1). This indicates that vaccination has a protective effect that is reduced following coadministration. Such observations have not been documented before, although some other studies reported increased reactogenicity for some of these coadministrations. We found a reduced protective effect for fever and neurological events following DTaP/IPV/Hib+MenC, and fever after DTaP/IPV/Hib+RV. Other studies assessing the safety of these coadministrations found no differences between coadministration and separate administration.16 45–47 Coadministering two vaccines led to less AEFI than expected based on the RIs after separate administration for 28% of analysed AEFI. The aforementioned literature review also found reports of such a inhibitory effect of vaccine coadministration on diarrhoea and fever following DTaP/IPV+RV,48 erythema following DTaP/IPV/Hib/HepB+MenC,49 and nasopharyngitis and insomnia following MMRV+PCV.50,16
Adding a fourth vaccine did not significantly alter the reactogenicity for the studied AEFI. To date, no other studies are available on the two unscheduled coadministrations of four vaccines included in our study.
Based on the RIR alone, our observations underpin the safety of coadministration of two scheduled routine paediatric vaccines. Our findings also indicate that adding a third vaccine may lead to a greater burden due to AEFI, in line with previous studies.16 Either way, we recommend further research into the severity of these events following separate versus coadministration for a more comprehensive assessment of the burden caused by these events and to evaluate whether the benefits of coadministration outweigh its risks. For example by augmenting routine data collection with questionnaires and/or other data sources, as has been conducted in influenza vaccination,51 and including supplementary data such as hospital admissions and deaths.
We found no indications that never recommended coadministrations per se are less safe than recommended coadministrations. Two recommended (DTaP/IPV/Hib+PCV, DTaP/IPV or dTaP/IPV+MMR) and four never recommended (DTaP/IPV or dTaP/IPV+Hib/MenC, DTaP/IPV or dTaP/IPV+PCV, MMR+Td/IPV, Td/IPV+HPV) coadministrations of two vaccines did not lead to more AEFI, which is in line with other studies’ findings.16 46 52–54 One recommended (DTaP/IPV/Hib+MenB + PCV) and one never recommended (DTaP/IPV or dTaP/IPV+MMR + Hib/MenC) did not increase AEFI either. However, one study reported more fever, a higher reactogenicity for local and general symptoms (irritability) after DTaP/IPV/Hib+MenB + PCV.55 Also the unscheduled addition of a fourth vaccine did not lead to more AEFIs and we found no studies reporting safety concerns. Nevertheless, unscheduled coadministrations happen occasionally and hence data on AEFI following such coadministrations may be too limited to identify significant differences between separate and coadministrations.
To the best of our knowledge, this is the first real-life data study comparing the safety of coadministering vaccines vs the safety of separately administering the same vaccines in two scenarios: administration as recommended in the immunisation schedule and never recommended. We chose the SCCS method to control for between-person confounders by comparing the risk and reference periods in each patient. We used a 42-day exposure period corresponding to risk periods commonly used in vaccine pharmacovigilance studies and appropriate for hypothesis generating studies since it reassures capturing nearly all AEFI.56 The SCCS method requires only cases to provide consistent estimates of the RI and controls implicitly for fixed confounders.29 31 SCCS estimate RIs, comparing the incidences of adverse events in exposure periods to unexposed periods within persons.31 This is particularly useful for studying vaccines with high coverage for which unvaccinated controls may be hard to find.31 However, no estimates of absolute incidence can be obtained.29 Therefore, we recommend researchers to compare the incidences between separate and coadministration on the same data using other methods. The large quantity of real-life vaccination and event data allows for powerful analyses. However, data from medical records may be prone to misclassification and heterogeneous as they are recorded by different persons to document and inform medical practice and not specifically for this study. The data may be prone to reporting bias because parents may consult their GP related to AEFI differently than when such events would manifest without prior vaccination, which may lead to lower RIs. Relying on existing medical records limits analysis to the availability of variables captured in the database.57 Consequently, we invite researchers to replicate this study by using the same method but on different data from other sources. Given the emerging insights on non-specific effects of vaccinations and calls for studying the influence of the order of vaccinations on such effects,58 we advise to widen the research focus to address the potential influence of vaccine coadministrations on such non-specific effects as well.
The implementation of coadministration practices should be supported by evidence that coadministered vaccines are at least equally safe as separately administered vaccines. Real-life data show that coadministrations of two vaccines have an equal or even better safety profile than administering the respective vaccines separately, but adding a third vaccine can increase the incidence of AEFI. We call for enhanced surveillance for a more comprehensive evaluation of the risks associated with vaccine coadministrations, and whether such risks are outweighed by the benefits of coadministration.
Data availability statement
Data may be obtained from a third party and are not publicly available. Data used for this study remains stored on secure servers of the Oxford-Royal College of General Practitioners (RCGP) Research and Surveillance Centre (RSC), and can be accessed on the RCGP RSC conditions.
Ethics statements
Patient consent for publication
Ethics approval
This research was exempt from ethical approval. The research proposal and data request were evaluated and accepted by the RCGP RSC. No other approvals were required.
References
Footnotes
Handling editor Seye Abimbola
Contributors JB planned, designed, conducted the study and analyses, wrote the manuscrip, and is the guarantor; SdL and NK served as scientific advisors and critically reviewed the manuscript, YGW served as scientific advisor for the SCCS method and critically reviewed the manuscript; JB planned this study, served as scientific advisor, critically reviewed the study proposal and manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.