Article Text
Abstract
Introduction Estimating COVID-19 cumulative incidence in Africa remains problematic due to challenges in contact tracing, routine surveillance systems and laboratory testing capacities and strategies. We undertook a meta-analysis of population-based seroprevalence studies to estimate SARS-CoV-2 seroprevalence in Africa to inform evidence-based decision making on public health and social measures (PHSM) and vaccine strategy.
Methods We searched for seroprevalence studies conducted in Africa published 1 January 2020–30 December 2021 in Medline, Embase, Web of Science and Europe PMC (preprints), grey literature, media releases and early results from WHO Unity studies. All studies were screened, extracted, assessed for risk of bias and evaluated for alignment with the WHO Unity seroprevalence protocol. We conducted descriptive analyses of seroprevalence and meta-analysed seroprevalence differences by demographic groups, place and time. We estimated the extent of undetected infections by comparing seroprevalence and cumulative incidence of confirmed cases reported to WHO.PROSPERO: CRD42020183634.
Results We identified 56 full texts or early results, reporting 153 distinct seroprevalence studies in Africa. Of these, 97 (63%) were low/moderate risk of bias studies. SARS-CoV-2 seroprevalence rose from 3.0% (95% CI 1.0% to 9.2%) in April–June 2020 to 65.1% (95% CI 56.3% to 73.0%) in July–September 2021. The ratios of seroprevalence from infection to cumulative incidence of confirmed cases was large (overall: 100:1, ranging from 18:1 to 954:1) and steady over time. Seroprevalence was highly heterogeneous both within countries—urban versus rural (lower seroprevalence for rural geographic areas), children versus adults (children aged 0–9 years had the lowest seroprevalence)—and between countries and African subregions.
Conclusion We report high seroprevalence in Africa suggesting greater population exposure to SARS-CoV-2 and potential protection against COVID-19 severe disease than indicated by surveillance data. As seroprevalence was heterogeneous, targeted PHSM and vaccination strategies need to be tailored to local epidemiological situations.
- COVID-19
- systematic review
- epidemiology
- serology
- public health
Data availability statement
Data are available in a public, open access repository. Primary seroprevalence estimates and statistical code relevant to the study are available at the following GitHub link: https://github.com/serotracker/Africa-SRMA-seroprevalence/tree/ee308719ad22f5761ff7f5be884c1e0bfd743e78. Early results submitted via the Unity Study Collaborators initiative are available on the following Zenodo link: https://zenodo.org/communities/unity-sero-2021?page=1&size=20.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
There is limited published evidence on the seroprevalence of SARS-CoV-2 in Africa, including one previous systematic review and meta-analysis in the general population for the continent and global systematic reviews that under-represent studies in Africa due to sparse data.
Recently, in part via WHO’s Unity studies, the quantity and quality of available seroprevalence data has increased, providing the opportunity to understand the true extent of exposure to SARS-CoV-2 in Africa, disaggregated by demographic groups, place (eg, subregion, country) and time.
WHAT THIS STUDY ADDS
Our results indicate a high seroprevalence in Africa (65.1%) in July–September 2021, which had increased from 3.0% in April–June 2020, and large, persistent under-ascertainment of infection based on confirmed case-based data.
Our results also indicate considerable heterogeneity in seroprevalence within countries and between countries and African subregions.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
High, heterogeneous seroprevalence in Africa highlights the need for targeted serosurveillance, public health and social measures and vaccination strategies tailored to the local context, particularly to address geographic and demographic vulnerabilities.
Introduction
Africa is experiencing unprecedented challenges due to the COVID-19 pandemic, both directly and from the compounding effects of other health, economic and social factors.1 2 As of 9 February 2022, 11.1 million COVID-19 cases and 238 845 deaths were confirmed in the African continent.3 4 To date, the pandemic has progressed in four main waves, with the most recent wave largely due to the Omicron variant circulating in a number of countries. However, reported data indicate a less severe disease profile in Africa compared with other regions globally: fewer cases, proportionally fewer patients with severe outcomes and death and proportionally more asymptomatic cases.3 5 6
Precisely estimating COVID-19 cumulative incidence in Africa remains problematic due to challenges in contact tracing, routine surveillance systems and laboratory testing capacities and strategies in many countries. Furthermore, Africa is a large, complex and heterogeneous continent with a range of different economies, countries impacted by humanitarian crises, vulnerable population groups and unique public health challenges. With low vaccine coverage in Africa (17% as of 9 February 2022),7 using seroprevalence data to understand the true dynamics of SARS-CoV-2 infection and case underascertainment is key to map the extent of protection in the general population and inform the public health response.
SARS-CoV-2 seroprevalence studies in Africa have been under-represented in previous systematic reviews and meta-analyses due to sparse seroprevalence data. The one previous meta-analysis in Africa8 pooled results from studies published to April 2021, which included only a small number of studies (n=23 studies) with limited geographical coverage and scope (no nationwide studies). Seroprevalence data are now emerging from completed field investigations, in part enabled by the support of the WHO’s Unity Studies. At the start of the pandemic, Unity Studies developed a population-based, age-stratified seroepidemiological investigation protocol (to estimate seroprevalence, named herein as the SEROPREV protocol) and supported countries to plan and implement robust and standardised seroprevalence studies and to analyse and publish their results.9 These studies provide us with the opportunity to understand SARS-CoV-2 epidemiology in Africa despite low vaccination coverage and case underascertainment. To note, our investigative team has also conducted an analysis of studies aligned with the SEROPREV protocol globally, including regional comparison, which serves as the foundational source for the methods and analyses further developed in this paper10 to provide more detailed analyses for Africa.
We aimed to better understand SARS-CoV-2 epidemiology in Africa up to and before the emergence of the Omicron variant. We undertook a meta-analysis of population-based seroprevalence studies that were aligned with WHO’s standardised SEROPREV protocol to estimate SARS-CoV-2 seroprevalence in Africa to inform evidence-based decision making on public health and social measures (PHSM) and vaccine strategy. Our primary objectives were to: (1) estimate SARS-CoV-2 seroprevalence and changes over time in Africa as a whole and by UN subregions, (2) estimate the extent of undetected infections by comparing seroprevalence and cumulative incidence of confirmed cases and (3) identify heterogeneity in seroprevalence attributable to demographic factors, country-level factors and study design.
Methods
Search strategy, selection criteria and data extraction
We conducted a systematic review of seroprevalence data sources in the African continent published from 1 January 2020 to 30 December 2021, reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline (online supplemental appendix S1). We searched Medline, Embase, Web of Science and Europe PMC for published articles, preprints, grey literature and media reports (online supplemental appendix S2). To increase representativeness, we also accepted early results from ongoing studies in Africa shared by study teams with WHO via a standardised template11 up to 5 May 2022, which have been made available on an open-access repository.12 This review is registered with PROSPERO (CRD42020183634) where further details on our search strategy, inclusion criteria, screening and extraction protocol are available.13
Supplemental material
Full details on our inclusion and exclusion criteria are described elsewhere.10 Briefly, we included SARS-CoV-2 seroprevalence studies in the general population aligned with the WHO SEROPREV protocol9 10 conducted in the African continent. We included cohort and cross-sectional study designs. We also specified assay performance criteria for inclusion of at least 90% sensitivity and 97% specificity determined by the manufacturer. Exceptions to the assay performance criteria were made to accommodate resource limitations in COVID-19 emergency humanitarian settings (herein referred to as humanitarian response plan (HRP) settings or status, defined by the Global COVID-19 Humanitarian Response Plan).14 We excluded studies without a clear numerator or denominator; without study dates and seroprevalence estimate; studies sampling closed populations (prisons, schools, etc); and studies that excluded participants with previous COVID-19 diagnosis or vaccination. Sources identified through our search and results submitted directly to us through the Unity Studies Initiative were screened using the same selection criteria.
Screening was conducted in two stages by two independent reviewers: title and abstract screening, followed by full-text screening. Conflicts were resolved by a third reviewer. Articles selected for inclusion were then extracted and verified by two reviewers. In cases where sources contained multiple primary estimates of seroprevalence (ie, non-overlapping populations, separate methodologies, etc), the source (full text) was split into multiple individual studies for extraction. Reviewers collected information on the source’s key characteristics (journal/venue, publication date), study information (demographics, sampling criteria, testing strategy) as well as seroprevalence estimates.
Risk of bias assessment
All studies were critically appraised using a modified Joanna Briggs Institute (JBI) nine-point checklist for seroprevalence studies.15–17 Each study’s JBI rating was completed by two independent reviewers. Conflicts were resolved by consensus. An automated decision rule based on combinations of each reviewer’s JBI checklist was applied to determine overall study risk of bias (low, moderate or high). This automated decision rule is described in detail elsewhere.18
Data synthesis and analysis
Seroprevalence studies were classified by study design, sample frame, sampling method, type of serological assay and geographic scope (local, subnational or national) (online supplemental table S1). We further classified local scope into national capitals and other cities or towns over or under 300 000 inhabitants (online supplemental table S2), based on urban agglomerations defined by the UN.19 Countries were classified according to the UN’s African subregions,20 HRP status,14 and World Bank income level. Each round of cohort or repeated cross-sectional studies were classified as separate studies. Where there were multiple estimates per study unrelated to time, we prioritised by adjustment, antibody isotypes, test type and antibody targets (full details: online supplemental appendix S3.1). To best reflect the period covered by the estimate, we anchored each estimate to the date halfway between start of sampling and end (‘sampling midpoint date’). We also identified studies with seroprevalence estimates by urban and rural areas within cities or towns (as defined by study authors), males and females and 10-year age groups up to 60 years and over. Data were analysed using R statistical software V.4.1.2.21 There was no public or patient involvement in this research.
We summarised the characteristics of all identified studies (dataset 0). We used studies rated low or moderate risk of bias to estimate seroprevalence in the general population over time and conduct subgroup meta-analysis and meta-regression (dataset 1) and national studies rated low or moderate risk of bias to calculate seroprevalence to cumulative incidence ratios (dataset 2). We summarised the proportion of seropositives in each individual study (dataset 1) by country and over time, highlighting the scope of the result and which results derived from the same cohort or repeated cross-sectional data sources. We summarised other relevant variables in each country, including smoothed daily cases, cumulative incidence of cases reported to WHO3 and relative variant genome frequency shared via the GISAID initiative.22 To estimate combined seroprevalence from infection or vaccination in the general population, we meta-analysed reported seroprevalence overall and by UN subregion and quarter of sampling midpoint date (period of three calendar months ending on 31 March, 30 June, 30 September or 31 December) to reduce heterogeneity between studies using a random-effects model (metaprop in R). We used the Clopper-Pearson method to produce 95% CIs reflecting uncertainty.23 24 To estimate seroprevalence attributable to infection only, we adjusted reported seroprevalence using a standard formula before pooling studies.25
We also estimated the magnitude by which confirmed SARS-CoV-2 cases underestimated the true burden of disease. To do so, we applied our overall meta-analysis estimates of seroprevalence from infection to the African population26 to estimate true infections and divided them by the number of laboratory confirmed cases at the time.3 To examine differences by country, we calculated the ratio between estimated seroprevalence from infection and the cumulative incidence of COVID-19 cases for each national study in dataset 2. Finally, to put the ratios into context of different health systems across countries, we calculated Pearson’s correlation coefficient between the underascertainment ratios in each study and four indices of national health system functionality (access, quality, demand and resilience).27 To estimate asymptomatic seroprevalence, we summarised the proportion of seropositives that reported no COVID-19 symptoms during the study period in the aggregated results shared by Unity collaborators. We tested for differences in the distribution across age and sex groups using the Kruskal-Wallis (KW) H-test.
To explore possible causes of heterogeneity among study results, we conducted subgroup analysis and meta-regression. First, to quantify demographic differences in seroprevalence, we calculated the seroprevalence ratio between subgroups within each study with available data in dataset 1. We compared each 10-year age group to adults 20–29 years, males to females and urban to rural areas. To produce summary estimates, we aggregated the ratios across studies using inverse variance-weighted meta-analysis. Heterogeneity was quantified using the I2 statistic. We also constructed a Poisson generalised linear mixed-effects regression to explore associations between seroprevalence and study and country factors.28 Independent categorical predictors were selected a priori as UN subregion, sample frame, geographic scope, low or high population density in the study setting (thresholds and sources in online supplemental appendix S3.2) and type of serological test. Cumulative incidence of confirmed cases was selected as an independent continuous predictor.
Our main analysis used seroprevalence estimates uncorrected for test characteristics. As a sensitivity analysis, we also produced results adjusting for test characteristics through Bayesian measurement error models, with binomial sensitivity and specificity distributions. The sensitivity and specificity values for correction were prioritised from the WHO SARS-CoV-2 Test Kit Comparative Study conducted at the NRL Australia,29 followed by a multicentre evaluation of 47 commercial SARS-CoV-2 immunoassays by 41 Dutch laboratories,30 and from independent evaluations by study authors where author-designed assays were used.
Results
Selection of studies
We identified 73 348 titles and abstracts in our search (figure 1). Of these, 4221 full-text articles were included in full-text screening. We identified 56 data sources reporting studies aligned with the SEROPREV protocol in the African continent, 42 published and 14 aggregated results from collaborators, which contained a total of 153 unique seroprevalence studies (detailed information on each study: online supplemental tables S3–S5).
PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Study characteristics and quality assessment
The 153 identified studies represented 43% (23/54) of WHO African Continent Member States (MS).26 The data included 9 of 18 MS in Eastern Africa, 1 of 5 MS in Southern Africa (South Africa), 7 of 16 MS in Western Africa, 5 of 9 MS in Middle Africa, 1 of 6 MS in Northern Africa (Egypt) and 59% (n=17/29) of vulnerable HRP countries (online supplemental appendix S4, figure S1). Early results from Unity study collaborators in nine countries made up over one-third (35%, n=53/153) of identified studies.
Among the 153 studies included in the descriptive analysis (table 1, column 1), 26% (n=40) reported results at a national level and 7% at a subnational level. The remaining two-thirds of studies reported results at a local level, with 26% in the national capital, 7% in another major city over 300 000 inhabitants and 32% in a smaller city or town under 300 000 inhabitants. Of studies reporting results at a local level, 36% indicated the city was selected because of suspected high SARS-CoV-2 transmission, 62% mentioned convenience and 65% mentioned high population density.
Study characteristics
Half of the studies used probability sampling (51%) and most sampled blood donors (44%, n=68) or households (46%, n=70). The remaining studies sampled pregnant or parturient women (4%, n=6) and residual sera from outpatient clinics or primary healthcare facilities (6%, n=9). The most common study design was repeated cross-sectional (59%). Three studies in three countries (Burkina Faso, Mali and South Africa) used a longitudinal cohort design with probability sampling. Among the testing strategies used to measure seroprevalence, most studies used the Wantai SARS-CoV-2 Total Ab ELISA procured by WHO for standardisation (28%, n=43), other ELISA (33%, n=50) or chemiluminescent immunoassay (CLIA) (24%, n=37) assays. Sixteen studies used a lateral flow immunoassay (LFIA) (10%). Six studies (4%) had more than 5% of the national population vaccinated by the sampling midpoint. The most frequent risk of bias rating was moderate (40%, n=61), followed by high (37%, n=56) and low risk of bias (24%, n=36). Risk of bias ratings for individual studies are reported in online supplemental table S6.
Ninety-seven studies with low (37%, n=36) or moderate (63%, n=61) risk of bias (dataset 1) were included in the subsequent results.
Seroprevalence and its geographic and temporal variation
Seroprevalence ranged from 0% to 87% in all studies. For countries in Eastern and Southern Africa, this range was 0% in a national blood donor study in Malawi conducted in January 202031 to 73% in a local household sample in Addis Ababa, Ethiopia in April 2021.32 For countries in Western and Middle Africa, this was 3% in a national household sample in Sierra Leone, March 2021,33 to 87% in a national household sample in Gabon, December 2021.34
There was considerable variation in reported seroprevalence in the 20 countries in dataset 1 (online supplemental figure S2). Countries with notable cohort or repeated cross-sectional studies (Malawi, Kenya, Ghana and South Africa) are highlighted in figure 2, indicating sharp increases in seroprevalence in the general population over time (figure 2).
Reported seroprevalence, variants of concern and cumulative incidence by selected countries over time, March 2020–December 2021. Plots for all countries are reported in online supplemental figure S2. Top panel, left axis: shaded areas represent the relative frequency of major variants of concern (VOCs) circulating, based on weekly counts of hCoV-19 genomes submitted to GISAID (Global Initiative on Sharing Avian Influenza Data) that we have aggregated by month. Weeks with fewer than 10 total submissions in a given country were excluded from the analysis. Top panel, right axis: daily confirmed cases reported to WHO on a national level per million people, smoothed using local regression (Locally Estimated Scatterplot Smoothing, LOESS). Middle panel: each point is an individual seroprevalence study, and identical shapes represent studies originating from the same cohort or repeated cross-sectional data source. Bottom panel: cumulative incidence of confirmed cases per 100 people.
Pooled seroprevalence from infection or vaccination (random-effects model)
In the random-effects model, the pooled seroprevalence from infection or vaccination across Africa rose from 3.0% (95% CI 1.0% to 9.2%) (number of samples=6, I2=96.0) as of Q2 2020 to 65.1% (95% CI 56.3% to 73.0%) (n=8, I2=98.6) as of Q3 2021.
Subregional meta-analyses were conducted for each quarter from Q2 2020 to Q3 2021, the period with available data (figure 3 and online supplemental table S7). Pooled seroprevalence was 70.1% (95% CI 64.6% to 75.1%) (n=1) in Eastern Africa, 56.1% (95% CI 44.6% to 66.9%) (n=4) in Southern Africa, 73.3% (95% CI 64.2% to 80.9%) (n=2) in Western Africa and 75.5% (95% CI 72.4% to 78.3%) (n=1) in Middle Africa as of Q3 2021.
Pooled seroprevalence from infection or vaccination (random-effects model) by UN subregion and quarter, Q3 2020–Q3 2021. Point estimates and 95% CIs (error bars) are reported for each quarter, as well as the number of samples pooled (n).
Ratios of seroprevalence to cumulative incidence
There was considerable variation by country in the ratios of seroprevalence from infection (based on national studies) to cumulative incidence (online supplemental table S8). Ratios across all studies ranged from 18:1 to 954:1, with the highest underascertainment from 2020 Q4 to 2021 Q3 found in Nigeria (954:1, July 2021) and Malawi (696:1, October 2020) and the lowest in Sierra Leone (57:1, March 2021) and South Africa (18:1, March 2021). The ratios were moderately negatively correlated with health system access (Pearson’s r=−0.70), demand (−0.60) and resilience (−0.60). Health system quality was weakly negatively correlated with underascertainment (−0.26). Applying our overall estimated seroprevalence from infection to the African population suggests that true infections were 100 (95% CI 83 to 115) times larger than confirmed cases as of September 2021 (827 million estimated infections compared with 8.2 million confirmed cases).
Demographic, study and country factors associated with seroprevalence
The percentage of seropositive individuals who did not report symptoms prior to sampling (asymptomatic seroprevalence) overall and by age and sex subgroups for 15 studies reporting symptoms are shown in online supplemental figure S3. Asymptomatic prevalence in Africa was 71.0% overall (IQR 48.4%–80.8%, n=15). Median asymptomatic prevalence was similar across age groups, ranging from 53.2% in ages 60+ years to 71.3% in ages 20–29 years (KW H-test p=0.64). Median asymptomatic prevalence in males was 73.6%, compared with 68.1% in females (KW H-test p=0.51).
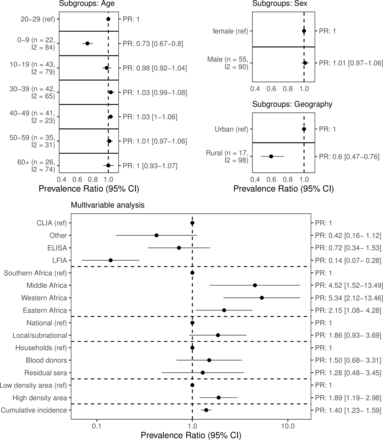
Within studies, seroprevalence was lower for children 0–9 years compared with aged 20–29 years (prevalence ratio (PR) 0.73 (0.67–0.80)). There were no differences between other age groups and 20–29 years, nor between males and females (figure 4). Compared with urban geographical areas, seroprevalence was lower for rural geographical areas (PR 0.60 (0.47–0.76)).
Factors associated with seroprevalence. Top panel: meta-analysis results. We calculated the ratio in prevalence between subgroups within each study then aggregated the ratios across studies using inverse variance-weighted random-effects meta-analysis. Heterogeneity was quantified using the I2 statistic. Each row represents a separate meta-analysis. Bottom panel: multivariable analysis using a Poisson generalised linear mixed-effects regression to model seroprevalence. CLIA, chemiluminescent immunoassay; cumulative incidence, cumulative incidence of confirmed cases per 100 people; ELISA, enzyme-linked immunosorbent assay; LFIA, lateral flow immunoassay; PR, prevalence ratio.
In the meta-regression, high population density was associated with an increase in seroprevalence compared with studies in areas of low population density (PR 1.89 (1.19–2.98)). Compared with Southern Africa, Eastern Africa (PR 2.15 (1.08–4.28)), Western Africa (PR 5.34 (2.12–13.46)) and Middle Africa (PR 4.52 (1.52–13.49)) were associated with higher seroprevalence. Higher cumulative incidence of confirmed cases was associated with an increase in seroprevalence (PR 1.40 (1.23–1.59)). Compared with studies that sampled households and communities, there were no differences between seroprevalence in studies that sampled blood donors nor residual sera. Finally, lateral flow assays were associated with lower seroprevalence compared with CLIA assays (PR 0.14 (0.07–0.28)), while there were no differences between ELISA nor other assays compared with CLIA assays, respectively.
Sensitivity analysis
Sensitivity analyses accounting for serological test performance from independent test kit evaluations showed no qualitative differences from the primary results (online supplemental figures S4–6). For example, overall seroprevalence in Q3 2021 using corrected estimates was 67.1% (58.5%–74.7%), compared with 65.1% (56.3%–73.0%) using uncorrected estimates.
Discussion
We estimate that overall seroprevalence is high in Africa (65.1% (56.3%–73.0%) in Q3 2021) and has risen considerably over time, from 3.0% (1.0%–9.2%) in Q2 2020, marked by sharp increases after the emergence of the Beta and Delta variants. Seroprevalence was highly heterogeneous both within countries—urban versus rural (lower seroprevalence for rural geographic areas), children versus adults (children aged 0–9 years had the lowest seroprevalence)—and between countries and African subregions (Eastern, Western and Middle Africa regions associated with higher seroprevalence). Our results suggest that the ratio of seroprevalence to cumulative incidence of confirmed cases is large (100:1 (83:1-115:1)) in Africa overall, varying from 18:1 to 954:1 by country over the study period.
Strengths and limitations
Previous assessments of COVID-19 seroprevalence studies highlight methodological heterogeneity as a key barrier to synthesising data.35–37 The dataset meta-analysed here is standardised, representative and granular, enabling unique insights into extrapolating seroprevalence in Africa. Recently, in part via WHO’s Unity Studies Initiative, more seroprevalence data have become available, disaggregated by demographic groups (age, sex), place (eg, subregion, country) and time (quarterly periods). In line with the equity principles of the Unity Studies Initiative, our dataset 1 included a broad range of studies in low-income countries (56%, n=54), lower middle income countries (LMICs) (23%, n=22) and vulnerable HRP countries (53%, n=51). Over one-third of studies were conducted at the national level, which is unique to this analysis. Unity study collaborators shared evidence, facilitating geographic coverage, timeliness, and reducing publication bias. Additionally, standardised epidemiological and serological methods (including the supply of a well-performing assay to LMIC) enabled through the Unity Studies Initiative means that the estimates included in our meta-analysis are robust and comparable. Finally, recognising that assay performance is a key determinant of seroprevalence in light of no gold standard for SARS-CoV-2 antibody testing, we linked our data to independent test kit evaluations29 30 of serological assay performance to correct seroprevalence estimates in a sensitivity analysis, helping ensure the robustness of our results to this source of bias.
This review should be considered in light of its limitations. First, while our dataset is more representative than studies published early in the pandemic, our meta-analysis estimates are extrapolated to the African continent from countries with seroprevalence studies and should be interpreted with some caution. In certain quarters, estimates were driven by countries with a disproportionate number of studies, often in the same population over time, in Eastern Africa (Ethiopia: 17 studies, Malawi: 20 studies), Southern Africa (South Africa: 20 studies) and Western Africa (Ghana: 4 studies, Nigeria: 7 studies). Other subregions were under-represented due to scarce data (eg, no studies in North Africa, five studies in Middle Africa). Second, many local studies (29% in a city/town <300 000 people) were included in our estimates of seroprevalence over time (dataset 1). These studies may not reflect the seroprevalence across an entire country: local studies are often conducted in large, dense and interconnected urban centres to investigate suspected high transmission or for convenience. Indeed, our results show that local urban areas are associated with higher seroprevalence estimates. Third, our results are based only on studies aligned with the SEROPREV protocol, and these may differ if using studies with other criteria. The SEROPREV protocol is broad, requiring a defined population, age standardisation and a robust test, which tend to be indicators of higher quality seroprevalence studies. Based on studies identified through SeroTracker’s living systematic review, only 11 general population studies in Africa at low or moderate risk of bias were excluded from this study because they did not meet the eligibility criteria, and thus would likely have minimal impact on the main findings.38 Fourth, we did not account for antibody waning, so the present study likely underestimates the true extent of past infection; similarly, case underascertainment is likely to be underestimated. Finally, due to delays in releasing seroprevalence study results, we could not produce estimates for Q4 2021, including since the emergence of the Omicron variant.
Methodological quality of the included studies
We evaluated study risk of bias using a validated, seroprevalence-specific tool based on the JBI Checklist for seroprevalence studies18 and found that 37% of studies were at high risk of bias. The most common reasons for this included an unrepresentative sample frame, non-probability sampling and not adjusting estimates for population characteristics. We found a higher proportion of high-risk studies in our study compared with another systematic review and meta-analysis in Africa (26%) by Chisale et al.8 The interpretation of items in the JBI checklist is subjective and can vary considerably within and between study teams. Moreover, Chisale et al. evaluated overall risk of bias by summing each item’s score; the automated decision rule in our tool puts more weight on items more likely to bias results, such as whether a representative sample frame was chosen and a well-performing antibody test was used.18
To minimise the risk of bias in our summary estimates, we included only studies at low/moderate risk in our meta-analysis of seroprevalence in the general population over time, subgroup meta-analysis and meta-regression, and case ascertainment estimates. Despite this effort, there are still methodological differences between the meta-analysed studies that may reduce their comparability. For example, one fifth of studies in our analysis dataset (19%, n=18/97) were convenience samples, which are less representative than population-based probability samples. To limit this bias, we required SEROPREV-aligned convenience samples to have a clearly defined sample frame (ie, sampling of volunteers excluded).
Context
The pooled seroprevalence in Africa estimated in this study (65.1% in Q3 2021) is among the highest in the world (comparable to the Southeast Asia region).10 With vaccination coverage in Africa being low during the study period (6.8% as of September 2021),7 this was mostly driven by infections. Our time-specific and region-specific estimates shed light on the trajectory of the pandemic. Of interest, we observe sharp increases in seroprevalence in 2021 in certain countries and regions, signifying the considerable number of infections caused by more transmissible variants22: for example, an increase from 11% (December 2020) to 65% (April 2021) seroprevalence in Malawi following the emergence of the Beta variant, and 26% (May 2021) to 60% (September 2021) in rural South Africa following Delta variant emergence. While case counts also increased during this time, this systematic comparison of seroprevalence data from different countries demonstrates the inexorable spread of infection in regions with limited or variable PHSM39 and vaccination roll-out, particularly where highly transmissible variants are circulating.
One possible explanation for high seroprevalence is potential cross-reactivity of antibodies against Plasmodium falciparum or common cold coronaviruses (CCCs). In areas of Africa with a high incidence of malaria,40 41 malaria cross-reactivity could follow two potential mechanisms: (A) SARS-CoV-2 antibody tests falsely reacting in malaria hyperendemic areas,42 or (B) cross-protection through CD8+ T cell activation from P. falciparum antigens.43 Broad anti-CCC antibodies have been identified following SARS-CoV-2 infection and vaccination.44 45 The exact role of cross-reactivity on seroprevalence estimates warrants further investigation.
In multivariable analysis, seroprevalence was heterogeneous between subregions: higher in Eastern, Western and Middle Africa compared with Southern Africa. The exact reasons for this heterogeneity remain unknown but could be related to mitigation strategy, health infrastructure and the effectiveness of PHSM implementation. The capacity to isolate has been shown to vary greatly in Africa,46 and challenges have been reported with social distancing in the Western and Middle subregions, especially in high density areas.47 48 This is also consistent with findings by Chisale et al.,8 who observed higher seroprevalence in studies conducted in Central Africa compared with other regions.
We observed heterogeneity within countries by age group and urban/rural geography. We observed lower seroprevalence in children 0–9 years, perhaps attributable to milder infections in this group, which are associated with lower antibody titres,49 and school closures, which have been common and lengthy in some African countries.50 In contrast to our global analysis,10 we did not find lower seroprevalence in adults 60+ years compared with adults 20–29 years. These results may be due to increased intergenerational mixing at the household level, as prior research in Africa has shown that households sharing space with persons aged 60+ years may have increased transmission risk.46 We also found lower seroprevalence was associated with rural geographical areas compared with urban areas, in line with other hypotheses and modelling associating rural areas with a potentially lower spread of infection due to decreased population density.51
The use of LFIA assays was associated with lower seroprevalence estimates compared with CLIA assays in the meta-regression. This may be explained by the lower sensitivity of LFIA, which can lead to more false negatives and underestimated seroprevalence.52
We observed greatly varying ratios between seroprevalence to cumulative confirmed case incidence by country, ranging from 18:1 in South Africa to 954:1 in Nigeria. The large fraction of asymptomatic cases in Africa is one possible reason for this; our estimates suggest that 71.0% (IQR 48.4%–80.8%) of cases have no symptoms, which accords with previous work documenting higher rates of asymptomatic infections in Africa compared with other regions.37 53 Underascertainment is well documented in African countries with low capacity for surveillance and laboratory testing,5 and indeed, lower levels of underascertainment were observed in countries with higher health system functionality indices. We estimate larger ratios compared with other parts of the world during the same period.10
Implications for practice, policy and future research
In contrast to routine surveillance data that rely on case reports, recently available seroprevalence studies have provided a more accurate understanding of the true extent of SARS-CoV-2 infection across Africa, amid low vaccine coverage. There is a need to strengthen surveillance infrastructure for priority diseases, including collaborative government researcher efforts and timely reporting mechanisms. Seroprevalence data need to be used alongside other sources of epidemiological data for policy decision making. This will collectively inform effective and tailored disease prevention control programmes, which must be deployed alongside other investments in public health and health system strengthening (eg, trained and motivated health workers, a well-maintained infrastructure and a reliable supply of medicines and technologies) to support their implementation and uptake in Africa for COVID-19 and other future or existing infectious disease threats.
The geographic distribution of early unpublished results shared with WHO for inclusion in this systematic review and meta-analysis demonstrates the need for standardised initiatives to help build enhanced surveillance and research capacity in LMIC. In this study, there were only two SEROPREV-aligned studies in Northern Africa (both in Egypt) identified here. Several populous and/or HRP countries lack study results to determine seroprevalence in the general population (eg, Ethiopia: no nationwide study results available since July 2020; Angola and the Democratic Republic of the Congo (DRC): no nationwide study results; Algeria, United Republic of Tanzania, Morocco, Somalia and Tunisia: no nationwide or local study results). Furthermore, there were no results available from island nations. We are aware of studies ongoing in some of these countries, emphasising the importance of open data practices, sharing early results, and collation and analysis of timely data. Improved quality and transparency of reporting studies would also help address this54: several studies were excluded from this systematic review and meta-analysis due to insufficient information (eg, no denominator stated, no end date stated, setting unclear).
Given the burden of SARS-CoV-2 varies across regions in Africa, there is also a need for studies representing different contexts (eg, heterogeneous access to health services, fragile environments, HRP countries) and vulnerable populations (eg, those with endemic infections like HIV and comorbidities, those living in high density urban settlements and refugee populations). Furthermore, there is a need to continue studying population differences in SARS-CoV-2 infection (eg, age, sex, geography, race, etc) to identify susceptible subpopulations to inform priorities for vaccination coverage and prevention and control measures. There is sparse data over time in many countries, which indicates a need to conduct more spatiotemporal studies (eg, longitudinal cohort, repeated cross-sectional). The use of well-designed convenience samples can help achieve this; for example, studies in blood donors represented over half of identified samples that used a cohort or repeated cross-sectional design (56%, n=70/124). Our results suggest that blood donors are a good proxy for the general population, as we found no statistical difference between seroprevalence estimates in blood donors and those in households and communities.
Population-based seroprevalence studies primarily give a reliable estimate of the exposure to SARS-CoV-2 infection in the general population. Where studies measure antibodies quantitatively, they can also estimate correlates for protection against infection.55 The risk of reinfection with the Omicron variant is reported to be much higher than for previous variants in both SARS-CoV-2 infected and vaccinated individuals.56 This implies that the presence of SARS-CoV-2 antibodies may no longer be a correlate of protection against infection for Omicron. However, seroprevalence estimates remain indicative of some protection against severe disease, as cellular immunity is unlikely to be affected even in the case of highly mutated variants such as Omicron.57 Research on the protective effectiveness of seroprevalence against disease severity, and the degree and durability of protection against Omicron, is limited and warrants further investigation.
Conclusion
This updated meta-analysis in Africa provides robust and representative seroprevalence results from over 40% of the continent’s MS, enabled through the standardisation and adaptability of the WHO Unity Studies. The substantial underascertainment of SARS-CoV-2 infection indicates that the majority of cases of SARS-CoV-2 in Africa are not captured by national surveillance systems, emphasising the continued need for comparable, aggregated and timely seroprevalence data that accounts for changing vaccination coverage. Our work provides a platform to develop Africa’s surveillance portfolio, building on existing local capacity to enable targeted and regular seroprevalence studies in a network of sentinel countries. This should focus on identifying susceptible populations and monitoring them over time for the prioritisation of PHSM and vaccination in-line with country policy.58 SARS-CoV-2 seroprevalence is high and heterogenous in Africa, suggesting greater population exposure to SARS-CoV-2 and potential protection against COVID-19 severe disease than has been previously indicated by confirmed case data and vaccine coverage. As such, PHSM and vaccination strategies tailored to local settings and specific populations are warranted.
Supplemental material
Data availability statement
Data are available in a public, open access repository. Primary seroprevalence estimates and statistical code relevant to the study are available at the following GitHub link: https://github.com/serotracker/Africa-SRMA-seroprevalence/tree/ee308719ad22f5761ff7f5be884c1e0bfd743e78. Early results submitted via the Unity Study Collaborators initiative are available on the following Zenodo link: https://zenodo.org/communities/unity-sero-2021?page=1&size=20.
Ethics statements
Patient consent for publication
Acknowledgments
We thank colleagues at WHO and partner organisations including Kathleen Gallagher, Amen Ben Hamida, Christopher Murrill, Toni Whistler, Venkatachalam Udhayakumar (US Centers for Disease Control and Prevention); Vincent Richard (Institut Pasteur and the Institut Pasteur International Network); Dr John N Nkengasong, Dr Mohammed Abdulaziz (Africa CDC); Issifou Alassani, Joël Béni-Victoire Anani, Selome Azanman, Magaran M Bagayoko, Fatoumata Binta Tidiane Diallo, Siaka Conde, Kokou Davi, Nollascus Ganda, Aboubacar Inoua, Bismwa Ruhana Mirindi, Berthe Njanpop, Solome Okware, Joseph Wamala (WHO Country Offices in the Africa Region) and Michael Ryan (WHO HQ); Robert Blanchard and the WHO/Dubai Logistics Team. SeroTracker (led by RKA, including MW, HW, ZL, XM, TY, CC, MY-L, JP, MPC, DB, ML, MS, GRD, NI, CZ, SP, HPR, TY, KCN, DK, SAA, ND, CD, NAD, EL, RKI, ASB, ELB, AS, JC) is grateful for support from WHO, Canada’s COVID-19 Immunity Task Force through the Public Health Agency of Canada, the Robert Koch Institute, and the Canadian Medical Association Joule Innovation Fund. RKA additionally thanks the Rhodes Trust for its support.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
HCL, HW, MW and LS are joint first authors.
JO, RKA and IB are joint senior authors.
Handling editor Senjuti Saha
Collaborators Unity Studies Collaborator Group: Rafiou Adamou (Centre de Recherche Médical de Lambaréné (CERMEL), Gabon); Ayola A Adegnika (Centre de Recherche Médical de Lambaréné (CERMEL), Gabon; Institut für Tropenmedizin, Eberhard Karls Universität Tübingen and German Center for Infection Research (DZIF), Tübingen, Germany); Samira Z Assoumou (Département de Bactériologie et de Virologie, Université des Sciences de la Santé, Gabon); Rosemary A Audu (Nigerian Institute of Medical Research, Nigeria); Jacob S Barnor (World Health Organization, Regional Office for Africa, Brazzaville, Congo); Enyew Birru (Ethiopian Public Health Institute, Addis Ababa, Ethiopia); Henry K Bosa (Ministry of Health, Uganda; Kellog College, University of Oxford, Oxford, UK); Emily L Boucher (Cumming School of Medicine, University of Calgary, Calgary, Canada; Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, UK); Annie Chauma-Mwale (Public Health Institute of Malawi, Ministry of Health, Malawi); Matthew P Cheng (Divisions of Infectious Diseases and Medical Microbiology, McGill University Health Centre, Montreal, Canada); Judy Chen (McGill University, Montreal, Canada); Cheryl Cohen (Center for Respiratory Disease and Meningitis, National Institute for Communicable Diseases, Johanessburg, South Africa; School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa); Tienhan S Dabakuyo-Yonli (Epidemiology and Quality of Life Research Unit, INSERM U1231, Georges François Leclerc Centre – UNICANCER, Dijon, France); Gabriel Deveaux (Cumming School of Medicine, University of Calgary, Calgary, Canada); Boly Diop (Surveillance Division, Prevention Directorate, Ministry of Health and Social Action, Dakar, Senegal); Titus H Divala (Kamuzu University of Health Sciences, University in Blantyre, Malawi); Emily K Dokubo (US Centers for Disease Control and Prevention, Atlanta, USA); Irene O Donkor (Noguchi Memorial Institute for Medical Research, Accra, Ghana); Claire Donnici (Cumming School of Medicine, University of Calgary, Calgary, Canada); Nathan Duarte (McGill University, Montreal, Canada); Natalie A Duarte (Faculty of Arts and Science, University of Toronto, Toronto, Canada); Didier K Ekouevi (Department of Public Health, University of Lomé, Togo); Paulin N Essone (Laboratoire National de Santé Publique, Libreville, Gabon); Timothy G Evans (COVID-19 Immunity Task Force, McGill University, Montreal, Canada); Lee Fairlie (WITS Reproductive Health and HIV Institute, Faculty of Health Sciences, University of the Witwatersrand, South Africa); Ousmane Faye (Virology Department, Institut Pasteur de Dakar, Dakar, Senegal); Anne von Gottberg (Center for Respiratory Disease and Meningitis, National Institute for Communicable Diseases, Johanessburg, South Africa; School of Pathology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa); Tiffany G Harris (ICAP, Columbia University, New York, USA; Department of Epidemiology, Mailman School of Public Health, Columbia University, USA); Natasha Ilincic (University of Toronto, Toronto, Canada); Elsie A Ilori (Nigeria Centre for Disease Control, Nigeria); Jackie Kleynhans (Center for Respiratory Disease and Meningitis, National Institute for Communicable Diseases, Johanessburg, South Africa; School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa); Dayoung Kim (Faculty of Science, University of Calgary, Calgary, Canada); Olatunji M Kolawole (Department of Microbiology, Faculty of Life Sciences, University of Ilorin, Ilorin, Nigeria; Ministerial Expert Advisory Committee on COVID-19, Health Sector Response, Federal Ministry of Health, Abuja, Nigeria); Jambo C Kondwani (Malawi-Liverpool-Wellcome Clinical Research Programme, Malawi; Liverpool School of Tropical Medicine, UK); Michael Liu (Harvard Medical School, Harvard University, Cambridge, USA); Emma Loeschnik (Department of Epidemiology and Biostatistics, Schulich School of Medicine and Dentistry, Western University, Ontario, Canada); Sheila Makiala-Mandanda (Institut National de Recherche Biomédicale, Kinshasa, Democratic Republic of the Congo; Université de Kinshasa, Democratic Republic of the Congo); Alexandre Manirakiza (Institut Pasteur of Bangui, Central African Republic); Pinyi N Mawien (Preventive Health Services, Ministry of Health, Juba, South Sudan); Portia C Mutevedzi (South African Medical Research Council Vaccines and Infectious Diseases Analytics Research Unit, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa; School of Pathology, Faculty of Health Sciences, University of Witwatersrand, South Africa); Jason M Mwenda (World Health Organization, Regional Office for Africa, Brazzaville, Congo); Paulin E Ndong (Laboratoire National de Santé Publique, Gabon); Edgard B Ngoungou (Département Epidémiologie et Bio statistique, Université Sciences de la Santé, Libreville, Gabon); Francine Ntoumi (Fondation Congolaise pour la Recherche Médicale, Brazzaville, Republic of Congo); Eric M Osoro (Washington State University, Global Health Kenya, Nairobi, Kenya; Paul G. Allen School of Global Health, Washington State University, USA); Samiratou Ouedraogo (McGill University, Montreal, Canada; National Public Health Institute, Burkina Faso); Sandrine L Oyegue (Centre Interdisciplinaire de Recherche Médical de Franceville (CIRMF), Gabon); Sara Perlman-Arrow (McGill University, Montreal, Canada); Jesse Papenburg (Montreal Children's Hospital, McGill University Health Centre, Montreal, Canada; Department of Epidemiology, Biostatistics and Occupational Health, McGill University, Montreal, Canada); Hude Quan (Department of Community Health Sciences, University of Calgary, Calgary, Canada); Hannah P Rahim (Boston Consulting Group, Boston, USA); Karampreet Sachathep (ICAP, Columbia University, New York, USA; Department of Population and Family Health, Mailman School of Public Health, New York, USA); Shobna Sawry (WITS Reproductive Health and HIV Institute, Faculty of Health Sciences, University of the Witwatersrand, South Africa); Mitchell Segal (University of Toronto, Toronto, Canada); Anabel Selemon (Cumming School of Medicine, University of Calgary, Calgary, Canada); Judith Shang (US Centers for Disease Control and Prevention (US-CDC), Cameroon); Joel F Djoba Siawaya (CHU Mère-Enfant Fondation Jeanne Ebori, Gabon); Kristen A Stafford (Center for International Health, Education, and Biosecurity, University of Maryland School of Medicine, USA; Division of Epidemiology and Prevention, Institute of Human Virology, University of Maryland School of Medicine, USA); Laura Steinhardt (US Centers for Disease Control and Prevention, Atlanta, USA); Cheikh Talla (Epidemiology, Clinical Research and Data Science, Institut Pasteur de Dakar, Senegal); Halidou Tinto (Institut de Recherche en Science de la Santé - Clinical Research Unit of Nanoro, Burkina Faso); Isidore T Traore (Programme de Recherche sur les maladies infectieuses, Centre MURAZ, Bobo-Dioulasso, Burkina Faso; Institut Supérieur des Sciences de la Santé, Université Nazi Boni, Bobo-Dioulasso, Burkina Faso); Joe A Theu (International Training and Education Center for Health (I-TECH), Malawi); Ines Vigan-Womas (Immunophysiopathology and Infectious Diseases Department, Institut Pasteur de Dakar, Senegal); Tyler Williamson (Department of Community Health Sciences, University of Calgary, Calgary, Canada); Tingting Yan (University of Toronto, Toronto, Canada); Cedric P Yansouni (Divisions of Infectious Diseases and Medical Microbiology, McGill University Health Centre, Montreal, Canada; J.D. MacLean Centre for Tropical Diseases, McGill University Health Centre, Canada); and Caseng Zhang (McMaster University, Hamilton, Canada).
Contributors An author reflexivity statement for equity in global health research was prepared and is included in the online supplemental appendix S5 of this article. Guarantor: HCL. Conceptualisation: HCL, IB, LS, TN, MK, RKA, NB. Data curation: HCL, AnV, LA, JO, TA, LL, AiV, HW, MW, XM, ZL, NB, CC, MY-L, ELB, JC, GRD, CD, ND, NAD, NI, DK, ML, EL, KCN, SP, HPR, MJS, AS, TY and CZ. Formal analysis: HW, RKA, DB, JP, MPC. Funding acquisition: IB, JO, MVK, RKA, NB and TY. Investigation: HCL, IB, LS, AN, MVK, BC, AnV, LA, AR, JO, TA, PW, LL, AiV, RAA, EB, AC, CC, TSD, BD, THD, EKD, LF, TGH, EAI, KCJ, JK, OMK, HKB, SM, AM, PCM, EMO, SO, IOD, KKS, SS, KAS, LS, AOT, CT, JAT, HT, ITT, IV, AvG and CPY. Methodology: HW, RKA, DB, NB, IB, HCL, AN, MV, MVK, CC, MPC, DC, CD, ND, ML, JP, MY-L, TW, TE and HQ. Project administration: HCL, IB, JO, MVK, MW, NB, RKA, TY, RAA, PNA, TB, JSB, BLH, OMK, PCM, JMM, AN, NN, JDS, LS, AOT, HT, MV, AvG and CPY. Resources: HCL, LS, BC, AnV, LA, AR, JO, TA, LL and AiV. Supervision: IB, AnV, LA, AR, JO, TA, PRW, LL, RP, MVK, DB, MPC, JP, RKA, NB and TY. Writing – original draft: HCL, IB, LS, AN, HW, MW and RKA. Writing – review and editing: all authors. All authors debated, discussed, edited and approved the final manuscript. All authors had full access to the full data in the study and accepted responsibility to submit for publication.
Funding WHO COVID-19 Solidarity Response Fund and the German Federal Ministry of Health COVID-19 Research and Development Fund. SeroTracker (led by RKA, including MW, HW, ZL, XM, CC, MY-L, DB, JP, MPC, ML, MS, GRD, NI, CZ, SP, HPR, TY, KCN, DK, SAA, ND, CD, NAD, EL, RKI, ASB, ELB, AS, JC and NB) report funding from WHO, Canada’s COVID-19 Immunity Task Force through the Public Health Agency of Canada, the Robert Koch Institute and the Canadian Medical Association Joule Innovation Fund.
Competing interests WHO had a role in study design, data collection, data analysis, data interpretation and writing of the report. No other funders had any such role. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. Authors were not precluded from accessing data in the study, and they accept responsibility to submit for publication. Potential other competing interests of named coauthors include: RKA reports personal fees from the Public Health Agency of Canada and the Bill and Melinda Gates Foundation Strategic Investment Fund, as well as equity in Alethea Medical (outside the submitted work). MPC reports personal fees from GEn1E Lifesciences (outside the submitted work), nplex biosciences (outside the submitted work) and Kanvas biosciences (outside the submitted work). JP reports grants from MedImmune (outside the submitted work) and Sanofi-Pasteur (outside the submitted work), grants and personal fees from Merck (outside the submitted work) and AbbVie (outside the submitted work) and personal fees from AstraZeneca (outside the submitted work). CCo reports funding from Sanofi Pasteur (outside of the submitted work). KE reports being cochairman of ANRS group on public health and social science in France (outside of the submitted work). JDS reports consulting fees from ASLM, GIZ health-focus and l’Oréal (all outside of the submitted work).
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.