Article Text
Abstract
Introduction Tuberculosis (TB) is a global health emergency and low treatment adherence among patients is a major barrier to ending the TB epidemic. The WHO promotes digital adherence technologies (DATs) as facilitators for improving treatment adherence in resource-limited settings. However, limited research has investigated whether DATs improve outcomes for high-risk patients (ie, those with a high probability of an unsuccessful outcome), leading to concerns that DATs may cause intervention-generated inequality.
Methods We conducted secondary analyses of data from a completed individual-level randomised controlled trial in Nairobi, Kenya during 2016–2017, which evaluated the average intervention effect of a novel DAT-based behavioural support programme. We trained a causal forest model to answer three research questions: (1) Was the effect of the intervention heterogeneous across individuals? (2) Was the intervention less effective for high-risk patients? nd (3) Can differentiated care improve programme effectiveness and equity in treatment outcomes?
Results We found that individual intervention effects—the percentage point reduction in the likelihood of an unsuccessful treatment outcome—ranged from 4.2 to 12.4, with an average of 8.2. The intervention was beneficial for 76% of patients, and most beneficial for high-risk patients. Differentiated enrolment policies, targeted at high-risk patients, have the potential to (1) increase the average intervention effect of DAT services by up to 28.5% and (2) decrease the population average and standard deviation (across patients) of the probability of an unsuccessful treatment outcome by up to 8.5% and 31.5%, respectively.
Conclusion This DAT-based intervention can improve outcomes among high-risk patients, reducing inequity in the likelihood of an unsuccessful treatment outcome. In resource-limited settings where universal provision of the intervention is infeasible, targeting high-risk patients for DAT enrolment is a worthwhile strategy for programmes that involve human support sponsors, enabling them to achieve the highest possible impact for high-risk patients at a substantially improved cost-effectiveness ratio.
- Treatment
- Tuberculosis
- Health policy
Data availability statement
Data are available on reasonable request. Deidentified subject level data set will be made available on case-by-case basis on reasonable request to the corresponding author for research purposes on publication.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
The WHO promotes digital adherence technologies (DATs) as facilitators for improving tuberculosis treatment adherence.
Previous research on DATs has provided mixed results and it remains unknown if DATs create intervention generated inequality (IGI) by disproportionately benefiting patients who already have a low probability of an unsuccessful outcome.
WHAT THIS STUDY ADDS
The effect of the DAT on clinical outcomes was highly heterogeneous across patients.
The effects were largest for patients who are at a high risk of an unsuccessful treatment outcome.
Differentiated enrolment policies, targeted at high-risk patients, can increase the average intervention effect while simultaneously decreasing heterogeneity.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
DATs may not create IGI and have the potential to reduce inequity in outcomes.
Targeting DATs towards high-risk patients may improve population health outcomes and programme efficiency, particularly in settings with limited resources.
Introduction
Tuberculosis (TB) has been considered a global health emergency by the WHO since 1993, and TB elimination is a key target of the United Nations Sustainable Development Goals.1 TB remains a global health challenge with an estimated 1.5 million deaths in 2020. Despite the existence of effective treatment,2 successful treatment completion remains a challenge, and this exacerbates the epidemic by accelerating transmission and drug resistance.3–5 In most TB programmes, a subset of patients may have a particularly high probability of an unsuccessful treatment outcome, and therefore, be most in need of treatment adherence support (ie, high-risk patients). Achieving equitable outcomes—a stated objective of the WHO’s End TB Strategy6—requires interventions that have a demonstrated ability to reduce the probability of an unsuccessful treatment outcome in such high-risk patients.
The WHO currently promotes digital adherence technologies (DATs)—such as feature or smart phone-bases strategies, electronic pillboxes or ingestible sensors—as possible facilitators for improving TB treatment adherence in resource-limited settings.7–9 However, little research has investigated whether DATs are more likely to benefit high-risk patients or, alternatively, whether these technologies may be ineffective for such patients or even create intervention-generated inequality (IGI) by disproportionately benefiting patients who already have a low probability of an unsuccessful outcome (ie, low-risk patients).10 Some researchers have argued that technology-based health interventions may disproportionately benefit low-risk patients and create IGI, because such individuals may be more advantaged, and therefore, have higher baseline access to these technologies (eg, mobile phones) or greater ability to use them.10 On the other hand, if a technology disproportionately benefits high-risk patients, such an intervention could help achieve more equitable outcomes and improve health system efficiency, especially if it is specifically targeted to those patients.
Previous research on DATs in high TB burden settings has provided mixed results for average intervention effects. Furthermore, this literature has, to our knowledge, not examined the heterogeneity of intervention effects, the potential of IGI, or the potential of targeted enrolment. In high-quality randomised controlled trials (RCTs) in Pakistan and Uganda, DATs did not improve TB treatment outcomes for the overall patient population, which may also suggest these technologies were unlikely to have benefited high-risk patients.11 12 In India and Vietnam, suboptimal patient engagement with a feature phone-based intervention and electronic pillboxes raised concerns regarding the accuracy of these DATs for identifying nonadherence.13 14 Some of the challenges shaping technology nonengagement—including poor cellphone access or health literacy, stigma and frequent travel—may disproportionately affect high-risk patients.9 13 14 Indeed, a study conducted in Peru found that TB patients with poor cellphone access were more likely to have unsuccessful treatment outcomes.15 In contrast, a study conducted in South Africa found that use of an electronic pillbox successfully identified a subpopulation of patients who had challenges adhering to both drug-resistant TB therapy and HIV therapy.16 In the context of these limited and mixed findings, additional research is urgently needed to understand the heterogeneity of DAT-based TB interventions.
In this paper, we use data from a completed RCT17—which evaluated the average intervention effect of a novel DAT behavioural support system on unsuccessful treatment outcomes (a composite measure capturing death during TB treatment, treatment failure or lost to follow-up)—to train a causal random forest model18 to answer three research questions related to the equity impacts of the intervention. First, was the effect of the intervention heterogeneous across individuals? Second, was the intervention less effective for high-risk patients? Third, can differentiated care improve programme effectiveness and equity in treatment outcomes in settings where universal provision of the intervention is not possible? We find high variability in the intervention effect, with the greatest benefit accruing to high-risk patients. Differentiated provision of this DAT-based intervention has the potential to increase the average intervention effect while decreasing unsuccessful treatment outcomes in the overall TB patient population. As such, our findings have important implications for improving treatment outcomes while potentially increasing the efficiency of TB care delivery in low-resource, high TB burden settings.
Methods
Study setting
We conducted a secondary analysis of data from a completed individual-level RCT that was conducted in 17 health clinics in Nairobi, Kenya between 2016 and 2017. Kenya is listed among the 30 high burden TB states, with an estimated overall national TB prevalence of 426 cases per 100 000 population in 2016.19 Nairobi is the largest city in Kenya with a population of over 4 million. The estimated TB prevalence in urban areas of Kenya is higher than the national average, at 760 cases per 100 000 adult population. In 2020, Kenya reported a TB treatment success rate of 86%.20 Nairobi also had a similar treatment success rate. According to recent estimates, mobile phone penetration in Kenya is over 95%.21 22
Intervention design
The intervention, which was compared with the standard of care as part of the RCT, has four main components. First, patients received an SMS message every day, reminding them to take their medication. Second, patients were expected to verify their treatment adherence every day, using an unstructured supplementary services data (USSD) interface. Third, patients could use the USSD interface to access (A) educational information about TB and (B) information on their adherence performance compared with other (anonymised) patients. These features were selected based on principles from behavioural science in order to motivate the patient’s adherence to treatment.23 Fourth, the patients could interact with study team members (former patients who successfully completed TB treatment and were recruited as support sponsors for the RCT)—trained on the aforementioned behavioural science principles—for support and advice. The interaction with study team members constitutes a limited human resource that cannot be provided to all patients at scale, and therefore, motivates the need for a differentiated care strategy (ie, prioritising high-risk patients for human contact).
The protocol and outcomes of the original trial were reported previously: the intervention was estimated to reduce the proportion of patients with unsuccessful treatment outcomes by approximately two-thirds (see summary of RCT data collection and intervention design in online supplemental appendix 1).17
Supplemental material
Data
The original RCT identified 1882 eligible patients (patients of all ages undergoing TB treatment) but excluded 693 individuals because 337 did not meet the inclusion criteria of (1) being bacteriologically diagnosed with TB by smear microscopy, culture or GeneXpert, (2) communicating in either Swahili or English and (3) having access to a mobile phone; 9 declined to participate; and 347 could not be enrolled due to a study member not being on site for their medical appointment. The remaining 1189 patients were randomised into intervention (N=609) or control (N=580).
The primary trial outcome of the aforementioned trial was an unsuccessful treatment outcome, defined as a composite of death during TB treatment, treatment failure (ie, the patient’s sputum smear or culture was positive at month 5 or later), or lost to follow-up (ie, the patient interrupted treatment for ≥2 consecutive months). A successful treatment outcome was defined as either cure (ie, the patient’s sputum smear or culture was negative at month 5 or later) or treatment completion (ie, finished all prescribed medication).
Aside from the trial outcome and intervention assignment, we obtained data for 20 patient characteristics, which make up the independent variables in our analyses. These variables included a mix of demographic, physiological and medical history information. Patients were excluded from our analysis if they had a confirmed misdiagnosis, were transferred out of their clinic, or were missing data for any continuous feature. All categorical variables with more than one category were encoded using binary indicators for each category and missing values were encoded as a separate category.
Model
We trained a causal random forest using the grf package in R.18 We used 5000 trees and tuned all other hyperparameters using an internal cross-validation procedure (see model details in online supplemental appendix 1). All other model parameters were set to their default values. The primary assumption of a causal random forest is unconfoundedness, which requires that, conditional on observables, the assignment to the experimental conditions is random. The condition is satisfied, since patients were randomly assigned to the treatment and control groups. Furthermore, we verified this assumption using a propensity score histogram and tested the goodness-of-fit using the statistical test proposed by the creators (see model details in online supplemental appendix 1).18
We used the causal random forest to obtain out-of-sample estimates for four standard quantities of interest. First, we estimated the average intervention effect across all patients with a corresponding 95% CIs. Second, we estimated an individual intervention effect with a corresponding 95% CI for each patient, defined as  . Third and fourth, we estimated the probability of an unsuccessful treatment outcome, conditional on the patient’s covariates and the intervention group assignment. For each patient i, we define
. Third and fourth, we estimated the probability of an unsuccessful treatment outcome, conditional on the patient’s covariates and the intervention group assignment. For each patient i, we define  and
and 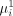 as the probability of an unsuccessful treatment outcome without intervention and with intervention, respectively. Note that the aforementioned quantities can be prospectively predicted for individuals based on sociodemographic and medical history information obtained through a questionnaire.
as the probability of an unsuccessful treatment outcome without intervention and with intervention, respectively. Note that the aforementioned quantities can be prospectively predicted for individuals based on sociodemographic and medical history information obtained through a questionnaire.
Was the effect of the intervention heterogeneous across individuals?
We used the estimated individual intervention effects ( ) and the corresponding 95% CI to determine if the intervention effects were heterogeneous across patients.
) and the corresponding 95% CI to determine if the intervention effects were heterogeneous across patients.
Was the intervention less effective for high-risk patients?
We conducted two analyses to address this question. First, we created a scatter plot and calculated Pearson correlation coefficient between the estimated intervention effect ( and the probability of an unsuccessful treatment outcome without intervention (
and the probability of an unsuccessful treatment outcome without intervention ( . Second, we confirm the first analysis using real trial outcomes by comparing the proportion of unsuccessful treatment outcomes between patients with a high and low estimated probability of an unsuccessful treatment outcome without intervention. To do this, we computed the median of
. Second, we confirm the first analysis using real trial outcomes by comparing the proportion of unsuccessful treatment outcomes between patients with a high and low estimated probability of an unsuccessful treatment outcome without intervention. To do this, we computed the median of 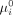 across all patients, denoted by
across all patients, denoted by  , and we partitioned the patient population into two groups: patients whose
, and we partitioned the patient population into two groups: patients whose  (high-risk patients) and
(high-risk patients) and  (low-risk patients). For each group, we computed the average intervention effect using the real outcomes from the RCT. In other words, we conducted a subgroup analysis where the two subgroups correspond to high-risk and low-risk patients.
(low-risk patients). For each group, we computed the average intervention effect using the real outcomes from the RCT. In other words, we conducted a subgroup analysis where the two subgroups correspond to high-risk and low-risk patients.
Can differentiated care improve programme effectiveness and equity in treatment outcomes?
In this context, differentiated care refers to a targeted enrolment strategy that prioritises high-risk patients. Prioritisation is necessary because the intervention involves a human component that cannot be scaled indefinitely. We consider a differentiated policy in which individuals are enrolled according to their predicted probability of an unsuccessful treatment without intervention ( . For this counterfactual policy, we ranked patients by their estimated
. For this counterfactual policy, we ranked patients by their estimated 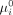 and enrolled individuals with the largest
and enrolled individuals with the largest  ’s. For a given level of enrolment capacity, we assess effectiveness (equity) by comparing the average (SD) of the individual intervention effects between differentiated enrolment and non-differentiated enrolment (ie, enrolment at random). For illustration, we first explored the impact of differentiated enrolment, assuming that the enrolment capacity is the same as in the RCT (ie, approximately half of the patient population). Second, we varied the enrolment capacity (captured by the proportion of patients enrolled) from 0 to 1, in increments of 0.1.
’s. For a given level of enrolment capacity, we assess effectiveness (equity) by comparing the average (SD) of the individual intervention effects between differentiated enrolment and non-differentiated enrolment (ie, enrolment at random). For illustration, we first explored the impact of differentiated enrolment, assuming that the enrolment capacity is the same as in the RCT (ie, approximately half of the patient population). Second, we varied the enrolment capacity (captured by the proportion of patients enrolled) from 0 to 1, in increments of 0.1.
Results
Data summary
A total of 1046 individuals were included in our analysis dataset. Table 1 provides a summary of all data used in our analyses, except for the categorical feature representing the TB clinic. Ultimately, 61 patients (12.4%) in the control group and 24 patients (4.6%) in the intervention group experienced an unsuccessful treatment outcome.
Patient characteristics stratified by the control group, the intervention/treatment group and total
The average intervention effect was positive
The average intervention effect was estimated to be 7.95 (95% CI 4.6 to 11.4), implying that, on average, the probability of an unsuccessful treatment outcome for an individual in the intervention group was approximately 0.08 less than an individual in the control group. The estimated average intervention effect is consistent with the results obtained using logistic regression in a prior analysis.17
The intervention effect was heterogeneous across individuals
Figure 1A displays a histogram of the individual intervention effects,  , which ranged from 4.2 to 12.4 with an average of 8.2. Figure 1B displays the sorted individual intervention effects with a corresponding 95% CI. The average interval width was 13.0 and the solid line corresponds to
, which ranged from 4.2 to 12.4 with an average of 8.2. Figure 1B displays the sorted individual intervention effects with a corresponding 95% CI. The average interval width was 13.0 and the solid line corresponds to 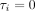 (ie, an intervention effect of zero). If a patient’s 95% CI intersects the solid line, then we cannot conclude that the intervention effect for that patient is statistically different from zero; 247 patients (24%) had a statistically insignificant intervention effect.
(ie, an intervention effect of zero). If a patient’s 95% CI intersects the solid line, then we cannot conclude that the intervention effect for that patient is statistically different from zero; 247 patients (24%) had a statistically insignificant intervention effect.
Causal forest estimates for (A) individual intervention effects and the average intervention effect (dashed line) and (B) the sorted individual intervention effects with 95% CIs.
The intervention helped those who needed it most
Figure 2A displays a scatter plot of the estimated intervention effect ( and the probability of an unsuccessful treatment outcome without intervention (
and the probability of an unsuccessful treatment outcome without intervention ( where patients are stratified according to their RCT group assignment. The strong positive relationship (Pearson correlation coefficient of 0.856 (95% CI: 0.839 to 0.871)) indicates that the treatment effect was largest for patients whose probability of an unsuccessful treatment outcome without intervention was high. Figure 2B displays histograms for the probability of an unsuccessful treatment outcome for the RCT group assignments; intervention in dark blue and control in orange. Under the counterfactual with no enrolment, the average estimated probability of an unsuccessful treatment outcome was 0.127 for the population. For comparison, the average probability of an unsuccessful treatment outcome was 0.128 for the control group (n=505) and 0.044 for the intervention group (n=541) in the RCT, corresponding to a population average of 0.085.
where patients are stratified according to their RCT group assignment. The strong positive relationship (Pearson correlation coefficient of 0.856 (95% CI: 0.839 to 0.871)) indicates that the treatment effect was largest for patients whose probability of an unsuccessful treatment outcome without intervention was high. Figure 2B displays histograms for the probability of an unsuccessful treatment outcome for the RCT group assignments; intervention in dark blue and control in orange. Under the counterfactual with no enrolment, the average estimated probability of an unsuccessful treatment outcome was 0.127 for the population. For comparison, the average probability of an unsuccessful treatment outcome was 0.128 for the control group (n=505) and 0.044 for the intervention group (n=541) in the RCT, corresponding to a population average of 0.085.
(A) displays a scatter plot of the estimated intervention effect and the probability of an unsuccessful treatment outcome without intervention with patients stratified according to their intervention group. (B) displays histograms for the probability of an unsuccessful treatment outcome conditional on the group assignment. (C) displays the proportion of unsuccessful treatment outcomes for the control and treatment group separated by high-risk and low-risk patients. The error bars represent the 95% CI and the numbers above the bars indicate the size of each group. The averages displayed on the x-axis denote the average predicted probability of an unsuccessful treatment outcome without intervention for each group.
Figure 2C displays the proportion of unsuccessful treatment outcomes in the control and intervention groups for high-risk and low-risk patients. The average intervention effect in the RCT was 10.9 (95% CI 5.8 to 16.0) and 5.4 (95% CI 1.0 to 9.8) for high-risk and low-risk patients, respectively. For patients in the control group, we find that the proportion of unsuccessful treatment outcomes was significantly higher for high-risk patients (0.157) as compared with low-risk patients (0.095) (difference=0.0654, p<0.001). For individuals in the intervention groups, the proportion of unsuccessful treatment outcomes was 0.041 and 0.048 (difference=0.007, p=0.69) for low-risk and high-risk patients, respectively.
Differentiated care reduces the mean and variation in unsuccessful treatment outcomes
Figure 3A displays histograms for the probability of an unsuccessful treatment outcome of the entire population (enrolled and non-enrolled) using differentiated (in blue) and non-differentiated (ie, enrolment at random, in orange) enrolment strategies, assuming the same enrolment capacity as the RCT. The average (SD) of the probability of an unsuccessful treatment outcome was 0.078 (0.034) and 0.085 (0.044) for differentiated and non-differentiated strategies, respectively. The average intervention effect for enrolled patients was 9.6 for the differentiated strategy, as compared with 8.1 for the non-differentiated strategy. Figure 3A provides a visual illustration of the drivers for the improved outcomes of the differentiated strategies, highlighting that the differentiated enrolment strategy focuses on enrolling patients with very high likelihoods of unsuccessful outcomes in the absence of intervention.
(A) Displays histograms for the probability of an unsuccessful treatment outcome of the entire population (enrolled and non-enrolled) using differentiated (blue) and non-differentiated (orange) enrolment. (B) Displays the estimated proportion of unsuccessful treatment outcomes and the corresponding intervention effect for enrolled patients under differentiated and non-differentiated enrolment policies with varying capacity. There are 95% CIs around each point.
Figure 3B displays the estimated proportion of unsuccessful treatment outcomes and the corresponding intervention effect (for enrolled patients) under differentiated and non-differentiated enrolment strategies with varying capacity. Differentiated enrolment strategies, targeted at high-risk patients increased the average intervention effect of DAT services by up to 28.5% (comparing the solid and dashed blue curves in figure 3B) while decreasing the population average and SD of the probability of an unsuccessful treatment outcome by up to 8.5% and 31.5%, respectively. Figure 3B highlights that the relative benefits of differentiated enrolment are highest when the enrolment capacity is the lowest.
Discussion
This study presents the first estimates of heterogeneity in the intervention effects of DAT support for treatment adherence among TB patients. Using data from a completed RCT, we confirm that the intervention effects are highly heterogeneous but also demonstrate that, in the case of this specific DAT-based intervention, the highest intervention effects are for high-risk patients, who had a high probability of an unsuccessful treatment outcome in the absence of adherence support. Specifically, we find that the estimated individual intervention effects range from 4.2 to 12.4, in terms of the percentage point reduction in the probability of an unsuccessful treatment outcome. Although the individual intervention effect is not statistically different from 0 for one-quarter of patients, for the overall population we find that the estimated individual intervention effects are strongly correlated with the probability of an unsuccessful treatment outcome in the absence of adherence support (our definition of high-risk patients). We confirm this result by stratifying the outcomes of the RCT, demonstrating that the average intervention effect for high-risk patients was significantly higher than for other (ie, low-risk) patients.
The potential impacts of technology-based interventions on health inequality has been a growing concern in the public health literature.10 24 25 Disadvantaged individuals may experience greater challenges with access to, adoption of or engagement with technology-based interventions.10 26 For the specific DAT-based intervention we evaluated, we find the opposite effect—the intervention disproportionately benefited disadvantaged, or high-risk, patients.
It is beyond the scope of our study to evaluate which component of Keheala’s intervention (ie, the reminders, the verification requirement, the educational information or the sponsor interaction) is driving the improved outcomes for high-risk patients, as the trial was designed to evaluate the overall impact of the suite of interventions.27 However, a potential explanation for why this DAT intervention performed better than others evaluated by RCTs in high TB burden settings,11 12 is the involvement of trained support sponsors who can assist patients with common adherence issues. The fact that recruiting and training support sponsors is the most resource intensive aspect of the intervention motivates our analysis to understand the potential benefits of differentiated care.
In addition to allowing DAT services to reach high-risk patients, differentiated enrolment can significantly impact the cost-effectiveness of such programmes. Since the DAT intervention we study had the highest impact on high-risk patients, the population-level impact of a scaled up intervention would be significantly higher with personalised enrolment than with uniform enrolment. Using the population of the RCT, our results demonstrate that personalising enrolment could increase the average intervention effect of the programme by up to 28.5% with no change in the number of enrolled patients and therefore no additional cost. These counterfactuals demonstrate that personalising treatment adherence support services by differentiating enrolment is not only important for high-risk patients but also policy makers who must prioritise cost-effective services given limited resources. In addition, the benefits of differentiated enrolment are highest in situations where the enrolment capacity is highly constrained, as might be the case for most countries with a high TB incidence.
Indeed, prior literature has called for increased personalisation in TB care, highlighting that technological advances may allow for the tailoring of treatment regimens to patients’ needs.28 Similarly, the highly variable risk estimates among patients with latent TB have been used to argue for differentiated and personalised strategies for initiating patients on preventive treatment.29 However, despite poor treatment adherence being a well-known barrier to improving TB care and despite this barrier being the focus of much research, little effort has been devoted to personalising treatment adherence support or incorporating differentiated care into DAT systems.9 30 Understanding individual intervention effects and using differentiated enrolment strategies is a first step towards shifting the current TB treatment adherence paradigm from observational (ie, monitoring and collecting data on patient adherence) to actionable (ie, combining behavioural data with analytics to improve patient adherence in a personalised manner).
Our study has at least two limitations. First, our results may not provide a complete picture of how this DAT-based intervention might impact treatment outcome equity across the broader TB patient population in Kenya or other high burden settings. For example, although mobile phone access is reported to be very high in Kenya and increasing in many other high TB burden countries, barriers to effective mobile phone access may still pose challenges in these settings. Similarly, for the purposes of our study, the term high risk has a precise definition and refers to the subset of the enrolled patient population who have a higher than median probability of a bad outcome in the absence of intervention. Therefore, our results should not be extrapolated to apply to patients who are underserved or at high risk for other reasons (including those patients who were excluded from the original study due to not speaking English or Swahili). Second, our findings may not be generalisable to other DATs, as Keheala is one of the few DAT-based interventions that has been shown to improve TB treatment outcomes via an RCT in a high TB burden country. However, the fact that Keheala’s average intervention effect has already been established motivates a closer look at the individual intervention effects studied in this paper. A broader implication of this paper is that following an RCT evaluating treatment adherence services, the appropriate course of action is to quantify individual intervention effects in addition to the standard average effects. Our results demonstrate that such analysis can inform the future scale-up of successful support programmes and policy makers should be aware of individual effects when deciding which DATs to adopt at scale.
More broadly, our results motivate a discussion of the scientific and ethical implications of using algorithms to preselect patients for higher intensity of intervention. From a scientific perspective, external validation of such algorithms is crucial, particularly if they are applied to a broader or different population from that used during training. From an ethical perspective, many would argue that if an intervention works, it should be offered to all patients. On the other hand, offering the intervention to all patients (including those with low risk of unsuccessful outcomes) will significantly increase its costs and thereby affect its cost-effectiveness. If machine learning-based preselection models can be shown to be accurate, they may enable policy makers to offer high impact services to the specific population that needs them the most.
Conclusion
In conclusion, we evaluated individual intervention effects of a DAT-based support system using data from a completed RCT. We found evidence of heterogeneity in intervention effects across patients. The individual intervention effects suggest that the DAT-based support system was most effective for high-risk patients, thereby closing inequity in treatment outcomes. We also demonstrated that differentiated care strategies—that is, targeted enrolment in the intervention—can benefit high-risk patients and improve overall treatment outcomes in situations when universal provision of the intervention is not possible.
Data availability statement
Data are available on reasonable request. Deidentified subject level data set will be made available on case-by-case basis on reasonable request to the corresponding author for research purposes on publication.
Ethics statements
Patient consent for publication
Ethics approval
The original trial was approved by the institutional review board of Kenyatta National Hospital and the University of Nairobi. The complete trial protocol is available at ClinicalTrials.gov number, NCT03135366. IRB review was not required for the work in the present paper because it does not involve human subjects as defined under 45 CFR 46.102(e).
Acknowledgments
The authors would like thank Dr. Enos Masini and Dr. Maureen Kamene, the former directors of the National Tuberculosis, Leprosy, and Lung Disease Program, for their assistance and support during the randomised controlled trial. The authors gratefully acknowledge the Keheala Support Sponsors, who make the difference in patients’ lives.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Handling editor Seye Abimbola
Contributors JJB (guarantor), JOOJ and EY conceptualised and designed the study. JJB and JOOJ wrote the manuscript and conducted the data analyses. JJB, JOOJ, EY and JR had full access to all the data in the study. All authors critically reviewed and revised the manuscript, including contributing to the interpretation of the results. All authors had final responsibility for the decision to submit for publication.
Funding The data used in this study were collected as part of a previous clinical trial that was made possible with support from the American people delivered through the US Agency for International Development (USAID).
Disclaimer The contents of this study do not necessarily reflect the opinion of USAID or the US Government. The funding source was not involved in the study design; collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Competing interests JR is the founder and chief executive of Keheala. The remaining authors have no conflicts of interest to declare.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.





