Article Text
Abstract
Background
Long-lasting insecticidal nets and indoor residual sprays have significantly reduced the burden of malaria. However, several hurdles remain before elimination can be achieved: mosquito vectors have developed resistance to public health insecticides, including pyrethroids, and have altered their biting behaviour to avoid these indoor control tools. Systemic insecticides, drugs applied directly to blood hosts to kill mosquitoes that take a blood meal, offer a promising vector control option. To date, most studies focus on repurposing ivermectin, a drug used extensively to treat river blindness. There is concern that overdependence on a single drug will inevitably repeat past experiences with the rapid spread of pyrethroid resistance in malaria vectors. Diversifying the arsenal of systemic insecticides used for mass drug administration would improve this strategy’s sustainability.
Methods
Here, a review was conducted to identify systemic insecticide candidates and consolidate their pharmacokinetic/pharmacodynamic properties. The impact of alternative integrated vector control options and different dosing regimens on malaria transmission reduction are illustrated through mathematical model simulation.
Results
The review identified drugs from four classes commonly used in livestock and companion animals: avermectins, milbemycins, isoxazolines and spinosyns. Simulations predicted that isoxazolines and spinosyns are promising candidates for mass drug administration, as they were predicted to need less frequent application than avermectins and milbemycins to maintain mosquitocidal blood concentrations.
Conclusions
These findings will provide a guide for investigating and applying different systemic insecticides to achieve more effective and sustainable control of malaria transmission.
- systemic insecticide
- malaria
- mosquito
- vector control
- computational modelling
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Key questions
What is already known?
Barriers to malaria elimination include mosquitoes forming resistance to insecticides used in long-lasting insecticidal nets (LLINs) and/or shifting their feeding patterns such that they avoid indoor control methods.
Ivermectin is a systemic insecticide that has been identified as a possible intervention method due to its mosquitocidal effect; however, over-reliance on a single drug should be avoided to minimise selection of resistant targets.
What are the new findings?
A number of systemic insecticides exist that should be considered for use in malaria control programmes already employing LLINs.
Isoxazolines and spinosyns are predicted to require less frequent dosing than avermectins (ie, ivermectin), thus making them operationally realistic choices to test for mass drug administration.
What do the new findings imply?
Systematic insecticides besides ivermectin could be used in tandem with LLINs to improve vector control strategies and reduce the transmission of malaria.
There is a critical need to further investigate these drugs’ safety and pharmacokinetics in relevant hosts and their effects on mosquitoes
Introduction
Long-lasting insecticidal nets (LLINs) and indoor residual sprays (IRS) have played significant roles in reducing the burden of malaria.1 2 However, several hurdles remain before elimination can be achieved. First, pyrethroids are heavily used in LLINs and, previously, IRS.3 As a result, the widespread and sustained use of this single class of insecticides has selected for mosquitoes that are resistant to the primary intervention methods.4 5 Second, because LLINs and IRS target mosquitoes that feed indoors on humans, mosquitoes have shifted their feeding patterns to avoid exposure. For instance, increasing numbers of mosquitoes have been found to seek their blood meals and/or rest outdoors after a new deployment of bed nets.6 Some malaria-transmitting mosquitoes avoid indoor interventions by obtaining blood meals from animal hosts.7 Though livestock cannot act as parasite reservoirs, bites diverted away from human hosts can act as temporary reprieve from insecticide exposure, increasing vector lifespans, and consequently, contributing to perpetuated transmission.8
To build on recent gains in malaria vector control, it is critical to develop a method that is effective against pyrethroid resistant, outdoor feeding/resting or plastically zoophagic mosquitoes.9 A promising solution is systemic insecticides, drugs that render host blood toxic to mosquitoes that take a blood meal.10 The types of systemic insecticides most relevant to treating mosquitoes are ectoparasiticides, drugs that target ectoparasites (eg, blood-feeding arthropods) and endectocides, drugs that target both endoparasites and ectoparasites. Many of these drugs have a mode of action distinct from pyrethroids, and thus should be effective against mosquitoes regardless of their susceptibility to pyrethroids.11–15 These systemic insecticides have been widely used to treat humans, livestock and domestic animals for infections ranging from gastrointestinal and systemic nematodes to blood-feeding parasites.16–19
Studies have demonstrated that mass drug administration (MDA) of the systemic insecticide ivermectin to humans and cattle can significantly decrease mosquito population numbers temporarily.20 21 To attain longer lasting impacts, the optimal use of systemic insecticides requires understanding their pharmacokinetics in the host to determine the dosing frequency necessary for maintaining lethal blood drug concentrations. Additionally, understanding the mosquito population’s feeding patterns will guide the decision of whether humans or animal hosts should be targeted. Finally, recent history has highlighted the importance of avoiding over-reliance on singular control tools; thus, it would be prudent to both investigate the effects of combining systemic insecticides with extant interventions and expand the arsenal of effective systemic insecticides from the current candidate of interest, ivermectin.22 23 Here, a review of systemic insecticides was conducted to collate the pharmacokinetic properties for different drug–host–route combinations. These data were then used to parameterise a mathematical model to illustrate the projected gains achievable in malaria control programmes already employing LLINs.
Methods
Patient and public involvement
Patients and the public were not involved.
Literature review
Veterinary and human parasiticides were identified with systemic properties that affected arthropods and were not prohibited nor being phased out of use in most countries. These included avermectins, milbemycins, neonicotinoids, spinosyns and isoxazolines. Unlike pyrethroids, which target voltage-gated sodium channels, avermectins and milbemycins target glutamate-gated chloride channels, neonicotinoids and spinosyns target unique sites of the nicotinic acetylcholinesterase receptor, and isoxazolines target γ-aminobutyric acid-gated chloride channels.11–15 Further discussion of the parasiticides’ structures and modes of action may be reviewed elsewhere.12–15
To determine the relevant pharmacokinetic studies for different systemic insecticide treatments, a review of the electronic literature was conducted. PubMed was searched from inception to 24 July 2018 using the following search terms: (“systemic insecticide” OR endectocide OR ectoparasiticide OR avermectin OR abamectin OR doramectin OR eprinomectin OR ivermectin OR selamectin OR milbemycin OR “milbemycin oxime” OR moxidectin OR isoxazolines OR afoxolaner OR fluralaner OR sarolaner OR lotilaner OR neonicotinoids OR imidacloprid OR nitenpyram OR spinosyns OR spinosad OR spinetoram OR “N-tert-butyl nodulisporamide”) AND (pharmacokinetics OR “area under the curve” OR kinetics OR “half-life” OR Cmax OR Tmax) AND (blood OR plasma). The complete list of accepted and rejected studies is available on request to the corresponding author.
Study inclusion was determined in two steps. First, the titles and abstracts were screened to determine studies that were irrelevant, not primary research (eg, letter or review), or purely computational. Irrelevant studies were defined as those focusing on drug mechanism, a drug that was not systemic or ineffective against mosquitoes, or hosts that are not targeted by mosquitoes for blood meals. After the initial screen, the full papers of the remaining studies were reviewed. Inclusion required the reporting of relevant pharmacokinetic parameters in plasma, use of a standardised drug, application of a single drug, and a test-population size n>3.
From the selected studies, the following data were extracted directly into an Excel spreadsheet: host studied, drug applied, dose applied, administration route, maximum drug concentration reached in the plasma (Cmax), area under the curve, absorption and elimination half-lives, mean residual time and volume of distribution. All data were summarised using basic descriptive statistics (mean and SD or SE) for each drug–host–route scenario. As different classes of drugs require different concentrations to achieve the same toxicity level, the doses for each combination of drug–host–route were not compared. Instead, the analysis focused on the pharmacokinetic metrics that impact the treatment’s efficacy and can be used to calculate the appropriate dose.
Data analysis
To determine the underlying trends between the pharmacokinetic parameters and each categorical factor (drug, host, administration route), a weighted (using the inverse of the variance) three-way analysis of variance (ANOVA) was conducted in Stata. Hosts with fewer than 10 observations were grouped into two categories based on bodyweight: other small (<75 kg) and other large (>75 kg).
Model development
To investigate strategies for applying different systemic insecticides to further limit malaria transmission, models from the literature were modified24–26 (see online supplementary text and table 1 for model development and parameter values). The proportion of bites that lead to infection, egg-laying rate and death rate of mosquitoes depend on the concentration of insecticide in LLINs (N) and systemic insecticides in livestock or humans (DL or DH, respectively) to which a mosquito is exposed.27–30 The model assumes the effects of the LLINs and systematic insecticides are additive and independent of each other’s concentration. Different host species and administration routes are characterised by different rates of adsorption, which were often not reported. Hence, to allow for comparison, the model represented the systemic insecticide’s pharmacokinetics as a single compartment and the initial insecticide concentration as the reported Cmax.
Supplemental material
Range of pharmacokinetic and pharmacodynamic parameters collected from the literature review (see online supplementary table 1 for more detail)
 (1)
(1)
 (2)
(2)
 (3)
(3)
 (4)
(4)
 (5)
(5)
 (6)
(6)
 (7)
(7)
The impact of insecticides used for livestock, human and bed net treatment diminishes as they degrade at rates  ,
,  and
and 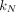 . These rates were determined by the half-lives recorded from the review. The transmission potential from vector to human or vice versa (bh and bm, respectively) is a function of biting frequency (a), the proportion of bites that successfully leads to infection in humans or mosquitoes (b and c, respectively), the coverage of bed nets (CN) and whether the LLIN’s insecticidal concentration is above the lethal concentration for killing 50% of the population in a set amount of time (
. These rates were determined by the half-lives recorded from the review. The transmission potential from vector to human or vice versa (bh and bm, respectively) is a function of biting frequency (a), the proportion of bites that successfully leads to infection in humans or mosquitoes (b and c, respectively), the coverage of bed nets (CN) and whether the LLIN’s insecticidal concentration is above the lethal concentration for killing 50% of the population in a set amount of time ( ). Mosquitoes lay eggs at a rate of
). Mosquitoes lay eggs at a rate of  , but this can be modified with the introduction of certain systemic insecticides (
, but this can be modified with the introduction of certain systemic insecticides ( ). Similarly, the mosquito natural death rate (μm) is modified with the introduction of LLINs and systemic insecticides (
). Similarly, the mosquito natural death rate (μm) is modified with the introduction of LLINs and systemic insecticides (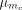 ). For both the egg laying and death rates, the impact of different control strategies depends on the proportion of bites on humans (
). For both the egg laying and death rates, the impact of different control strategies depends on the proportion of bites on humans (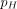 ), the coverage of LLINs and systemic insecticides in livestock or humans (CN, CL, CH, respectively), and the respective concentration thresholds for reducing fecundity or survival by 50% (
), the coverage of LLINs and systemic insecticides in livestock or humans (CN, CL, CH, respectively), and the respective concentration thresholds for reducing fecundity or survival by 50% ( , and
, and  ).
).
The relationship between mosquito fecundity or mortality and the concentration of LLINs or systemic insecticides is not linear, but is captured by Hill kinetics. The  ,
, 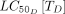 , and Hill coefficients (
, and Hill coefficients ( and
and  for egg-laying and death rates, respectively) were calculated for lab-reared Anopheles for different systemic insecticides by fitting a Hill equation to published data28–30 (online supplementary figures 1 and 2). The
for egg-laying and death rates, respectively) were calculated for lab-reared Anopheles for different systemic insecticides by fitting a Hill equation to published data28–30 (online supplementary figures 1 and 2). The  was calculated for wild Anopheles that displayed a range of resistance to LLINs, and the Hill coefficient (
was calculated for wild Anopheles that displayed a range of resistance to LLINs, and the Hill coefficient ( ) was calculated for laboratory reared, permethrin resistant Anopheles by fitting a Hill equation to published data.27 31 The time window (
) was calculated for laboratory reared, permethrin resistant Anopheles by fitting a Hill equation to published data.27 31 The time window (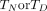 ) associated with each insecticide’s
) associated with each insecticide’s  was based on previously reported measurements.27–30
was based on previously reported measurements.27–30
Simulations
This model was used to explore the impact of combining different systemic insecticide treatments and LLINs on permethrin-resistant mosquitoes. A new LLIN was introduced every 3 years, as recommended by the WHO.32 Systemic insecticide treatments were designed to test a range of half-lives (0.1:100 days), dose concentrations (1:105 ng/mL), F50s (0:480 ng/mL) and 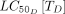 s (7:1180 ng/mL), based on data mined from the review. The simulation begins with the initial concentration of drug in the host’s blood, approximated by Cmax. The appropriate dose necessary to achieve Cmax can be calculated based on pharmacokinetics associated with each host species, as previously documented.33 Although many of the systemic insecticides identified in the review remain to be characterised as mosquitocidal candidates,
s (7:1180 ng/mL), based on data mined from the review. The simulation begins with the initial concentration of drug in the host’s blood, approximated by Cmax. The appropriate dose necessary to achieve Cmax can be calculated based on pharmacokinetics associated with each host species, as previously documented.33 Although many of the systemic insecticides identified in the review remain to be characterised as mosquitocidal candidates, 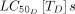 were identified from the avermectin, moxidectin, isoxazoline and spinosyn classes for Anopheles gambiae or Anopheles arabiensis and used to establish a range of realistic values.
were identified from the avermectin, moxidectin, isoxazoline and spinosyn classes for Anopheles gambiae or Anopheles arabiensis and used to establish a range of realistic values.  were only reported for a subset of avermectins and moxidectin. Each treatment characterised by half-life, Cmax,
were only reported for a subset of avermectins and moxidectin. Each treatment characterised by half-life, Cmax,  , and
, and  was dosed at frequencies ranging from weekly to annually. Strategy outcome was quantified by calculating the relative reduction in malaria prevalence after 3 years of drug treatment and LLIN coverage (
was dosed at frequencies ranging from weekly to annually. Strategy outcome was quantified by calculating the relative reduction in malaria prevalence after 3 years of drug treatment and LLIN coverage ( ) relative to 3 years of LLINs alone (
) relative to 3 years of LLINs alone (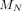 ).
).
 (8)
(8)
This framework was applied to evaluate strategies for areas with different levels of baseline malaria, mosquito populations with different feeding behaviours and different host coverage scenarios.
The livestock and human host coverage necessary to achieve a minimum 10% relative reduction in malaria for different dosing frequencies was estimated for each drug by holding the Cmax and half-life constant (online supplementary table 2). When available, the livestock values were based on topically or orally treated cattle and the human parameters were based on topically or orally treated ‘(other) small’ (the category humans were grouped into, due to small sample size). The exception is ivermectin, where the human host parameters were based on the weighted averages from studies of humans orally treated with ivermectin (Cmax=40.7 ng/mL, half-life=3.4 days). For drugs that were only characterised in dogs and small animals, those parameters were used for both human and livestock parameters.
Results
Data retrieval
From the initial 375 articles returned by the search, 237 full-text articles were assessed for eligibility, and 139 met the eligibility criteria (figure 1A). The studies reported pharmacokinetic parameters in 8 different host categories, 10 systemic insecticides and 6 administration routes (figure 1B–D). The three most studied hosts were cattle, sheep and dogs. Systemic insecticides studied included five avermectins (abamectin, doramectin, eprinomectin, ivermectin and selamectin), three isoxazolines (afoxolaner, fluralaner and lotilaner), one milbemycin (moxidectin) and one spinosyn (spinosad). These insecticides were applied intramuscularly, intraruminally, intravenously, orally, subcutaneously or topically. Note that intraruminal and intravenous routes are experimental and not currently operationally feasible; however, they were included to help determine the full range of action possible for each drug.
Identification of existing applications for systemic insecticides. (A) A review of PubMed identified relevant studies of existing systemic insecticides. (B–D) The included studies covered a range of different hosts, systemic insecticides and administration routes.
Data analysis
To evaluate the different treatment scenarios, weighted three-way ANOVAs were conducted for each pharmacokinetic parameter. Elimination half-life and Cmax were the only parameters with significant interactions (p<0.05) with host, route and drug. The significant effectors of half-life were drug class (p=0.016), route of admission (p=0.007), and interactions between host and route (p<0.001) and drug and route (p=0.007). Regardless of route of administration, the order of drugs from shortest to longest half-lives was avermectins<milbemycins<spinosyns<isoxazolines. The median half-life for avermectins and milbemycins was <10 days for all routes of administration, whereas isoxazolines’ half-life was >10 days (figure 2A, online supplementary figure 3). Comparing median half-lives for a given drug class across hosts showed some host dependency. For instance, milbemycins had a longer median half-life in dogs (19.4 days) than in other hosts (<10 days) and avermectins had a longer median half-life in cattle than in other hosts (figure 2B, online supplementary figure 4). When comparing administration routes across different hosts, topically applied drugs typically achieved longer half-lives than orally applied ones (figure 2C, online supplementary figure 5).
Half-life and Cmax are dependent on interactions between drug class, host and administration route. (A) Half-life is affected by the interaction between administration route and drug class. (B) The three most studied hosts show the effect of host and drug class on drug half-life. (C) The interaction between drug application route and host affects the drug half-life. (D) Cmax is affected by the interaction between host and drug. (E) The interaction between administration route and host also impacts Cmax. Drug classes: A, avermectin; I, isoxazoline; M,milbemycin; S,spinosyn; Routes: Im,intramuscular; Ir,intraruminal; Iv,intravenous; O,oral; S,subcutaneous; T, topical.
The significant factors for Cmax were drug (p=0.03) and interactions between host and drug (p=0.002) and host and route (p<0.001). The order of drugs from lowest to highest Cmax was different from that of half-lives: milbemycins<avermectins<isoxazolines<spinosyns (online supplementary figure 6). Cattle reported the lowest median Cmax for milbemycins, whereas dogs and sheep had the lowest Cmax for avermectins (figure 2D, online supplementary figure 7). There was also a dependency of Cmax on host and route (figure 2E, online supplementary figure 8). Although the intravenous route resulted in the highest Cmax for different hosts, due to the drug being directly delivered into the bloodstream, the order of resulting Cmax for other routes varied based on host.
The spread in half-lives and Cmaxs seen for a given drug–host–route combination can be attributed to host factors that may affect drug absorption and, consequently, the plasma concentration. These factors include age, gender, breed, diet, parasite infection, pregnancy, lactation, and whether topically treated hosts are restricted from self-licking.34–41 Understanding how host conditions affect basic pharmacokinetics is critical for designing optimal treatment strategies that can account for natural variations.
Basic dynamics of malaria transmission and control methods
The model captures the temporal dynamics of malaria transmission in a human population exposed to mosquitoes that bite humans and livestock indiscriminately. On the LLIN introduction, a general decline in malaria prevalence (symptomatic and asymptomatic individuals combined) is observed, followed by a steady increase as the insecticide in the net degrades and fewer mosquitoes are killed by LLIN exposure (figure 3A). The addition of treating one blood host population with systemic insecticide can further reduce malaria prevalence when applied at sufficient frequency.
Modelling malaria transmission and control methods. (A) The malaria prevalence ratio is compared for a population using LLINs alone (MN, black), LLINs with livestock treated yearly with systemic insecticide (MN+D1, red) and LLINs with livestock treated monthly with systemic insecticide (MN+D12, blue). (B) For a strategy using LLINs and livestock treated at a set dosing frequency (here, monthly), the relative reduction in malaria prevalence can be calculated for insecticides of various half-lives and Cmaxs. (C–I) The dosing frequency necessary to achieve a 10% relative reduction in malaria prevalence can be calculated for insecticides with different pharmacokinetic properties. Overlaying pharmacokinetic values gathered from the review predicts the minimum dosing frequency of existing systemic insecticides in certain host–route scenarios. Contour definitions from left to right: weekly, monthly, quarter-annually, biannually, annually. Here, we assume indiscriminate biting behaviour (ph=0.5). LLINs, long-lasting insecticidal nets.
Dosing strategy design and evaluation
To quantify the efficacy of different dosing strategies, the relative reduction in malaria prevalence after 3 years of using LLINs and applying systemic insecticides to one blood host population was compared with that of using LLINs alone. For a set dosing frequency, the relative reduction increased as a function of half-life and Cmax (figure 3B).
The minimum dosing frequency was calculated to achieve a target relative reduction (here, 10%) for different drugs distinguished by half-life, Cmax,  , and
, and  (figure 3C–I). The drugs with the longest half-lives and highest Cmaxs needed to be dosed the least often to maintain a sufficiently high concentration to remain lethal to feeding mosquitoes. Given the same half-life and Cmax, drugs with higher
(figure 3C–I). The drugs with the longest half-lives and highest Cmaxs needed to be dosed the least often to maintain a sufficiently high concentration to remain lethal to feeding mosquitoes. Given the same half-life and Cmax, drugs with higher 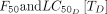 needed to be dosed more frequently to compensate for the decreased efficacy of drug on mosquito fecundity or survival.
needed to be dosed more frequently to compensate for the decreased efficacy of drug on mosquito fecundity or survival.
Overlaying the data gathered in the review for drugs with reported  s and
s and 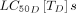 for Anopheles gambiae sensu lato predicted the frequency at which these existing drugs would need to be applied to achieve a 10% relative reduction in malaria prevalence. The avermectins, represented by ivermectin, eprinomectin and doramectin, have relatively low
for Anopheles gambiae sensu lato predicted the frequency at which these existing drugs would need to be applied to achieve a 10% relative reduction in malaria prevalence. The avermectins, represented by ivermectin, eprinomectin and doramectin, have relatively low  s and
s and 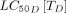 s, suggesting that relatively low concentrations of drug in the bloodstream would affect the fecundity and death rates of feeding mosquitoes. However, this impact is limited by these drugs’ relatively short half-lives, ranging from 0.4 to 11.1 days (table 1). Depending on the host and administration route, regimens with dosing frequencies ranging from weekly to quarter-annually would be required to achieve a 10% relative reduction in malaria prevalence. Although ivermectin and eprinomectin have a similar
s, suggesting that relatively low concentrations of drug in the bloodstream would affect the fecundity and death rates of feeding mosquitoes. However, this impact is limited by these drugs’ relatively short half-lives, ranging from 0.4 to 11.1 days (table 1). Depending on the host and administration route, regimens with dosing frequencies ranging from weekly to quarter-annually would be required to achieve a 10% relative reduction in malaria prevalence. Although ivermectin and eprinomectin have a similar  , ivermectin has a stronger effect on fecundity (online supplementary figure 2). Consequently, ivermectin would require less frequent dosing than eprinomectin to achieve the same target reduction. Doramectin has a lower impact on fecundity and death rates, with a higher
, ivermectin has a stronger effect on fecundity (online supplementary figure 2). Consequently, ivermectin would require less frequent dosing than eprinomectin to achieve the same target reduction. Doramectin has a lower impact on fecundity and death rates, with a higher  and
and  than ivermectin and eprinomectin.
than ivermectin and eprinomectin.
Although fluralaner and afoxolaner have higher 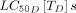 than ivermectin, they were predicted to achieve 10% relative reduction with yearly dosing, due to their longer half-life and higher Cmax. Similarly, spinosad has a high
than ivermectin, they were predicted to achieve 10% relative reduction with yearly dosing, due to their longer half-life and higher Cmax. Similarly, spinosad has a high  , a relatively long half-life of 11.3 days, a higher Cmax of 1550.0 ng/mL, and could achieve a 10% relative reduction when dosed biannually. These results are conservative, as the effect of fluralaner, afoxolaner, and spinosad on fecundity was assumed to be zero until it has been characterised in mosquitoes.
, a relatively long half-life of 11.3 days, a higher Cmax of 1550.0 ng/mL, and could achieve a 10% relative reduction when dosed biannually. These results are conservative, as the effect of fluralaner, afoxolaner, and spinosad on fecundity was assumed to be zero until it has been characterised in mosquitoes.
Despite some of the moxidectin studies reporting relatively long half-lives and high Cmaxs, its high 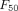 and
and 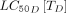 mean that it would have to be dosed more frequently (>weekly) or at higher doses to provide an effective complement to LLINs.
mean that it would have to be dosed more frequently (>weekly) or at higher doses to provide an effective complement to LLINs.
Application to different scenarios
This framework can predict the extent to which systemic insecticides could aid in the reduction of malaria prevalence in scenarios with different endemicities (online supplementary figure 9a–c). For instance, in a low-level mesoendemic environment (malaria prevalence=25%),42 LLINs alone played a significant role in reducing transmission; however, additional treatment of livestock and humans further reduced prevalence and could theoretically break transmission. In a high-level mesoendemic environment (malaria prevalence=50%), LLINs alone were not as effective and the additional treatment of livestock and humans could significantly reduce malaria transmission. When malaria is hyperendemic (prevalence=65%), the addition of systemic insecticide treatment reduced malaria prevalence relative to LLINs alone; however, to bring malaria transmission under control, longer, sustained treatment and/or the use of drugs with longer half-lives and higher Cmax, such as the isoxazolines, would be necessary.
Similarly, this framework can help evaluate control strategies for mosquitoes with different blood meal preferences (online supplementary figure 9d–e).43 Malaria transmission in regions with zoophilic mosquitoes was largely controlled by LLINs because the mosquitoes do not target humans as frequently. Treating livestock with systemic insecticides would be more effective than treating humans, requiring less frequent dosing for a drug with a given half-life and Cmax. Malaria in regions with anthropophilic mosquitoes was reduced the most with the treatment of both livestock and humans, with most of the reduction due to the treatment of humans. Dosing frequencies were increased, given the need to maintain high enough lethal systemic insecticide concentrations to affect a greater number of mosquitoes targeting humans for blood meals.
Estimating coverage
While 100% coverage is ideal, it is difficult and often unrealistic to achieve in practice. The minimum dosing frequency of each drug was calculated to achieve a relative reduction >10% for a range of humans and livestock coverage (figure 4). Intuitively, as the coverage of both hosts increased, the dosing frequency necessary to reach the target reduction generally decreased. Ivermectin treatment of human and cattle appeared to contribute equally to malaria reduction (figure 4A). If at least 50% of both humans and cattle were treated with ivermectin every 2 months, then at least a 15% relative reduction in malaria could be achieved over 3 years. If 75% of both humans and livestock were treated, then the dosing frequency could be reduced to once every 4 months and still achieve a 10%–12% relative reduction.
Effect of different coverage proportions in two hosts on the reduction in malaria prevalence after 3 years of treatment. (A–F) For each drug, the dosing frequency necessary to achieve at least a 10% relative reduction (RR) in malaria prevalence was calculated for a range of livestock and human coverages. The contour lines represent the minimum dosing frequency necessary to achieve the target reduction threshold. The colourmap indicates the level of relative reduction in malaria prevalence for a given coverage and dosing frequency. Here, we assume indiscriminate biting behaviour (ph=0.5).
Eprinomectin appeared less effective at reducing malaria than ivermectin, with the lowest frequency dosing option being every 2 months (figure 4B). This is largely due to ivermectin’s greater impact on fecundity (online supplementary figure 2). Assuming that the pharmacokinetic properties of eprinomectin in ‘small’ hosts are a proxy of those in humans, the malaria prevalence was more sensitive to increasing the eprinomectin coverage of humans than of livestock. This is because the eprinomectin reached a higher Cmax in humans than cattle (20.1 vs 11.0 ng/mL, respectively) and had a slightly longer half-life in humans than in livestock (4.8 vs 3.5 days) (online supplementary table 2). Thus, human blood meals would remain toxic to a mosquito for a longer period of time and could remain effective at lower dosing frequencies.
Doramectin was the least effective of the avermectins, requiring weekly dosing for the majority of the coverage scenarios to result in a 10% relative reduction in malaria prevalence (figure 4C). This is reflective of its  being larger than the Cmax.
being larger than the Cmax.
Assuming that the pharmacokinetic properties of both isoxazolines in dogs and ‘small’ hosts are a proxy of those in humans and cattle, fluralaner and afoxolaner were predicted to achieve the 10% reduction with yearly dosing and very low coverage (20% for fluralaner and 30% for afoxolaner) (figure 4D–E). With 70%–80% coverage of both populations, fluralaner was predicted to reduce malaria prevalence by 50%. However, afoxolaner applied at the same coverage would only reduce malaria prevalence by 25% because of its lower Cmax and higher  , relative to fluralaner. The isoxazolines’ Cmax is one to two orders of magnitude greater than the avermectins’, which results in the drug remaining at toxic levels in the blood for longer periods of time.
, relative to fluralaner. The isoxazolines’ Cmax is one to two orders of magnitude greater than the avermectins’, which results in the drug remaining at toxic levels in the blood for longer periods of time.
Assuming that spinosad’s pharmacokinetic properties in dogs can serve as a proxy for those in livestock and humans, it was also predicted to require less frequent dosing at lower coverage than the avermectins (figure 4F). Although spinosad had one of the highest Cmax values of the drugs simulated here, it also had one of the highest 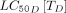 s. Thus, for a given coverage level, spinosad needed to be dosed more frequently than isoxazolines to reach the target reduction level.
s. Thus, for a given coverage level, spinosad needed to be dosed more frequently than isoxazolines to reach the target reduction level.
Moxidectin was not capable of reducing malaria prevalence, regardless of dosing frequency or host coverage, because its  is three orders of magnitude greater than its Cmax.
is three orders of magnitude greater than its Cmax.
Discussion
To date, ivermectin has been the main systemic insecticide considered for its mosquitocidal properties and, until recently, the only one marketed for human use.44–46 However, there are additional avermectins and different drug classes, such as milbemycins, spinosyns and isoxazolines, that should be screened as potential candidates for future mosquito control methods in livestock and/or humans. Here, through the combination of a review and a malaria transmission model, it was demonstrated how existing systemic insecticides can be applied to reduce malaria transmission in a range of scenarios. To make a 10% reduction in malaria prevalence beyond what is achieved by LLINs, some of the identified drugs need to be applied at frequencies much higher than the current MDA’s annual or biannual dosing regimen and at very high coverages.19 For instance, it was estimated that moxidectin would need to be dosed at an unrealistic frequency, more than once a week, to reduce malaria transmission. This supports studies that show moxidectin has little impact on mosquitoes and would unlikely be an effective choice for mosquito population control.28 Alternatively, isoxazoline, afoxolaner and spinosad are estimated to reduce malaria prevalence when applied once or twice a year with as little as 20%–30% (isoxazolines) or 60% coverage (spinosad).
The likelihood of these non-ivermectin drugs being administered to humans to improve vector control depends on their safety profiles as well as the regulatory and operational contingencies of conducting repeated MDAs. Moxidectin was approved in 2018 by the Food and Drug Administration to treat humans for onchocerciasis47; however, based on the above predictions, the dosing frequency necessary for moxidectin to make blood meals toxic to mosquitoes would be too high for an operationally feasible MDA. While isoxazolines remain to be tested in humans, Miglianico et al’s preliminary assessment of their safety anticipates that the prescribed human doses would be comparable to or lower than the concentrations reported to have no adverse effects in rats and dogs.30 It should be noted that, while isoxazolines are well tolerated by many cats and dogs, there have been instances of adverse events.48 49 Understanding individual-level risk factors for adverse effects will be important when characterising these drugs in new hosts and determining who to treat in MDAs. Based on the decades of repeated MDA of ivermectin in a range of settings, a biannual treatment strategy to improve vector control should be operationally feasible and could possibly be coordinated with existing campaigns.19
To reduce the dosing frequency of ivermectin and other drugs with relatively short half-lives, there are methods to attain higher concentrations of drug for longer periods of time. Recently, a study demonstrated the tolerability of high doses of ivermectin in humans and the resulting increase in time that the blood was lethal to mosquitoes.50 As an alternative to increasing the concentration of the delivered dose, there are adjuvants reported to improve the absorption or extend the half-life of systemic insecticides, such as lipids for lipophilic drugs or inhibitors for efflux pumps that remove xenobiotics, respectively.51–57 A third promising solution is the development of sustained-release devices, which have been shown to maintain a target concentration for 280 days in livestock.58
When designing an MDA to effectively reduce the transmission of a vectorborne disease, the secondary effects should also be considered.
Emergence of resistance
As the frequency of insecticide administration is increased to break the malaria transmission cycle, it is imperative to consider how these new treatment regimens will contribute to the emergence of resistance in mosquitoes and other parasites. Resistance is considered the greatest threat to malaria vector control.59 The two main mechanisms of insecticide resistance observed so far in mosquitoes can be categorised as metabolic or target site mutations.60 Studying how other arthropods have formed resistance to systemic insecticides will shed light on the potential pathways to resistance formation in mosquitoes. For instance, macrocyclic lactone resistance has been shown to arise in cattle ticks, fruit flies and body lice due to increased expression of adenosine triphosphate (ATP)-binding cassette transporter, P-glycoprotein and P450 genes.61–63 With strategic use of systemic insecticides that target different mechanisms, monotherapy could be avoided and the development of resistance in mosquitoes could be delayed.
Selecting for resistant, non-mosquito parasites is also a concern. One study reported ivermectin-resistant Rhipicephalus microplus found on 50% of cattle being treated regularly with ivermectin for gastrointestinal nematodes.64 Similarly, repeated treatment of ivermectin for onchocerciasis in humans has selected for resistant Onchocerca volvulus in Ghana.65 As these systemic insecticides are commonly used to control other parasite infections in humans and livestock, it is important to consider this in drug selection and actively screen for unintended consequences. Refugia, or leaving a small portion of the population untreated, offers a possible solution for reducing the selective pressure on parasites and arthropods to form resistance.64 66
Presence in food products
Considering how different systemic drugs are eliminated from a host’s system is also important in evaluating a new treatment programme. For instance, lactating hosts treated with a single dose of eprinomectin or ivermectin produced milk with detectable drug levels for weeks, thus exposing the nursing young.67 68 Due to the unknown effects of the drug on a newborn, nursing human mothers in the first week after delivery have been excluded from MDAs of ivermectin.69
Additionally, systemic insecticides from treated hosts becomes incorporated into dairy and meat products.70 Regulations have been established for levels of acceptable residues of a few drugs, such as eprinomectin; yet, most other drugs have not been licensed for use in dairy animals and do not have an acceptable limit for drug concentration found in milk.71 It was surmised that the extent of drug excretion and residence time in milk depends on a drug’s lipophilicity and route of administration.72 73 However, more studies are needed to characterise the different drugs’ excretion in milk and, subsequently, establish safety limits for suckling young or consumable items. In the meantime, refugia offers a possible solution to ensure that the meat and milk produced by a portion of the livestock are safe to consume.
Limitations
Since the model parameters were limited to the pharmacokinetic/pharmacodynamic values reported in the literature, there are a number of limitations to this approach. To achieve a large enough sample size, hosts were categorised by species. As a result, the impact of subspecies details, such as breed and health status, on pharmacokinetic properties were no longer distinguished. For instance, ivermectin was observed to be less available in zebu Gobra, a Western African cattle breed, than other cattle breeds74; however, the pharmacokinetic behaviour of doramectin and moxidectin were similar.75 Additionally, parasitised animals were reported to have faster absorption times and shorter circulation times for ivermectin than their healthy counterparts.38 Another constraint was being limited to pharmacokinetic data from one species as a proxy for how the drug might act in cattle or humans. Although interspecies variation in drug pharmacokinetics is significant, this was the most relevant data available for simulations until further studies are conducted. Furthermore, the effect of the isoxazolines and spinosyns on mosquito fecundity remains to be quantified. The proposed model conservatively assumed that those drugs had no effect on egg-laying rates. Finally, although lotilaner was identified as an isoxazoline with a particularly long half-life and high Cmax, its mosquitocidal effects remain to be measured and thus could not be included in the simulations. These limitations reveal the need to further investigate these drugs’ safety and pharmacokinetics in relevant hosts and their effects on mosquitoes. Nevertheless, this framework can help identify scenarios in which systemic insecticides should be considered for malaria control and develop an effective MDA strategy for a range of systemic insecticides.
Conclusions
Studies have demonstrated that systemic insecticides can significantly decrease mosquito population numbers temporarily. To design effective, long-term vector control strategies with systemic insecticides, their pharmacokinetics and pharmacodynamics need to be understood. Here, multiple systemic insecticides with different mechanisms of action and pharmacokinetic/pharmacodynamic characteristics that could be used in MDAs have been highlighted. The simulations provide a foundation for further characterising how wild mosquitoes respond to systemic insecticides. Given the history of mosquitoes forming resistance to the insecticides in LLINs and IRS, having a variety of systemic insecticide strategies that target different mechanisms is key to maximise sustainability of these promising, new methods.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Handling editor Alberto L Garcia-Basteiro
Contributors HM helped conceive the idea, designed the literature review, screened the papers, developed the model, ran the simulations, analysed the data and wrote the manuscript. LF-K helped analyse the literature review data and edit the manuscript. LY helped conceive the idea, interpret results and edit the manuscript.
Funding HM was funded by the Whitaker International Program. LF-K was supported by an Australian National Health and Medical Research Council Fellowship (APP1158469). LY was funded by the MRC (MC_PC_15097).
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available on reasonable request.






