Article Text
Abstract
Miltefosine, the only oral drug approved for the treatment of leishmaniasis—a parasitic disease transmitted by sandflies—is considered as a success story of research and development (R&D) by a public-private partnership (PPP). It epitomises the multiple market failures faced by a neglected disease drug: patients with low ability to pay, neglect by authorities and uncertain market size. Originally developed as an anticancer agent in the 1990s, the drug was registered in India in 2002 to treat the fatal visceral leishmaniasis. At the time, miltefosine was considered a breakthrough in the treatment, making it feasible to eliminate a regional disease. Today, access to miltefosine remains far from secure. The initial PPP agreement which includes access to the public sector is not enforced. The reality on the ground has been challenging: shortages due to inefficient supply chains, and use of a substandard product which led to a high number of treatment failures and deaths. Miltefosine received orphan drug status in the USA; when it was registered there in 2014, a priority review voucher (PRV) was awarded. The PRV, meant to facilitate drug development for neglected disease, was subsequently sold to another company for US$125 million without, to date, any apparent impact on drug access. At the heart of these concerns are questions on how to protect societal benefit of a drug developed with public investment, while clinicians worldwide struggle with its lack of affordability, limited availability and sustainability of access. This article analyses the reasons behind the postregistration access failure of miltefosine and provides the lessons learnt.
- leishmaniasis
- health policy
- public health
- treatment
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Summary box
Miltefosine is a major therapeutic advance as the only oral drug for leishmaniasis. Its development showed that public-private partnership (PPP) is a viable model for promoting research and development (R&D) in neglected tropical diseases (NTDs).
However, access to miltefosine postlicensure is limited. Low availability and affordability have been key issues globally, despite an agreement between the manufacturer and public institution(s).
PPPs focusing on product development for neglected and other diseases thus should aim, beyond the registration of the product, on the following:
Mechanism(s) to enforce framework and legal agreements between partners need to improve.
Ensuring access downstream is imperative: any new NTD tools being developed should include a postmarketing or postregistration access plan.
Drug pricing structures should be transparent: manufacturers should not take advantage of a monopolistic situation to overcharge.
Priority review voucher as an incentive to enhance R&D for NTD needs fixing; applicants should seek regulatory approval and demonstrate appropriate access strategies.
Introduction
Miltefosine, the only oral drug approved for the treatment of leishmaniasis, is an example of successful research and development (R&D) for a neglected tropical disease (NTD) that fails to reach the people who need it. Leishmaniases (infectious diseases caused by multiple species of Leishmania protozoan parasites and transmitted by the Phlebotomine sandfly) result in 700 000 to 1 million new cases annually worldwide.1 More than 1.5 billion people are at risk in 97 endemic countries.2 The disease is associated with malnutrition and immunosuppression as well as with poverty, poor housing and population displacement.3–6 The visceral form (kala-azar or visceral leishmaniasis, VL) is fatal when untreated. VL is the cause of the second largest parasitic disease burden after malaria. Each year, the infection causes 50 000–90 000 cases and 20 000–30 000 deaths.7 Stigma and disability due to cutaneous lesions and mucocutaneous form—involving the destruction of mucosa of nasopharynx—are devastating.8 9 The Indian subcontinent, eastern Africa and Brazil in Latin America are regions enduring a high burden. Transmission can be human to human, but animals are reservoir hosts in zoonotic areas such as southern Europe.10 In the absence of vaccines and effective vector/reservoir control, diagnosis and treatment remain the cornerstone of public health programmes in most parts of the world.11
Treatment options for leishmaniasis are limited.12 Medicines for such a disease are not attractive targets for the profit-driven pharmaceutical industry to invest their R&D efforts because most of the patients are poor. This situation has been described as an example of market failure, a modern welfare economy concept, defined as inefficient outcomes in markets where standard assumptions (perfect competition, symmetrical information) are non-existent or violated, leading to a net society loss.13 14 In the context of pharmaceutical R&D, the term has been aptly used.15–17 The main incentive for the producers—the ability to sell products at high prices—does not apply to NTDs, and market challenges are further compounded by perceived lack of intellectual property rights protection in developing countries. For more than 50 years, VL was treated with a single regimen—injectable pentavalent antimonials—until alarming failure rates and drug resistance were shown in India.18 Other medicines for leishmaniasis (amphotericin B, paromomycin, pentamidine or liposomal amphotericin) are all parenteral, toxic or too expensive. Thus a new, better drug was sorely needed.12
In 1995, the Special Programme for Research and Training in Tropical Diseases at WHO (WHO/TDR) engaged in a public-private partnership (PPP) with a pharmaceutical company, Asta Medica19 for the clinical development of miltefosine. This development involved repurposing what was originally an anticancer compound.20 21 Clinical trials proved miltefosine administered orally was superior to antimonial injections. In 2002, India’s Central Drug Standard Control Organisation approved miltefosine (Impavido) as the first-line regimen for the treatment of VL.22 This therapeutic breakthrough was a major factor behind the launch in 2005 of a VL elimination initiative on the Indian subcontinent. Oral administration enabled more patients to be treated in primary care settings.23 24 Subsequently, miltefosine was registered in various countries for both VL and cutaneous leishmaniasis (CL) and was included in the WHO Model List of Essential Medicines (EML) in 2011.25 26
Nonetheless, access to miltefosine after it was approved—the postlicensure or postregistration phase—has been less of a success story. The medicine never became as affordable and widely available as originally anticipated. The price of miltefosine made the medicine unaffordable for the majority of patients, most of them poor and marginalised.27 Even when provided for free by the public health system in the Indian subcontinent, the supply of the drug never quite met the demand.28 The Bangladeshi VL elimination programme opted for a locally sourced, less expensive alternative product. However, this generic version was clinically ineffective, and on verification, the capsules lacked the active pharmaceutical ingredient.29
To this day the drug remains valuable as a partner drug in combination regimens to treat VL and for several other clinical indications, yet miltefosine is hardly available in countries where leishmaniasis burden is high. Widespread adoption of miltefosine was challenging, due to various reasons that this paper attempts to unravel.
Fifteen years ago, WHO/TDR made a substantial R&D investment with a clear goal to reach people in need of life-saving medicine, yet access to this medicine remains compromised. We analyse the lessons learnt in the context of R&D for NTDs, the postlicensure phase and recommend strategies moving forward to increase access to this drug.
The development of miltefosine for leishmaniasis: a PPP success story
Miltefosine (hexadecylphosphocholine) is the only oral drug currently registered for the treatment of leishmaniasis.30 Two research groups discovered the compound in the early 1980s: one in Germany investigating the antitumour activity and another in the UK working on anti-inflammatory properties.21 31 Dose-limiting gastrointestinal adverse events in several phase I and II studies32 33 resulted in the discontinuation of the drug’s development as an oral drug for the treatment of solid tumours.20 Its development as a topical formulation for treating cutaneous metastases of breast cancer continued though, and Miltex (Bayer, UK) has been marketed in Europe since 1992.34–36 In 1987, miltefosine’s antileishmaniasis activity in vitro and in vivo was described.37 Excellent oral bioavailability in mouse models was found, in addition to superiority as compared with intravenous pentavalent antimonials in these animals.38 These results established miltefosine as a development candidate for the treatment of human VL. A proof-of-concept study conducted in India39 provided encouraging data for further clinical studies.40–42
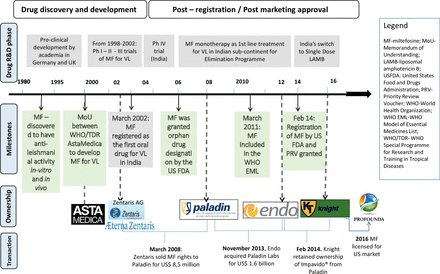
In 1995, WHO/TDR partnered with Asta Medica (later Zentaris, see figure 1), providing funding and expertise to further develop the drug for the treatment of VL.43 44 The motivation for the company in the partnership was linked to the potential market in South Asia, and substantial in-kind public input from WHO/TDR.45 Between 1996 to 2004, seven clinical trials were carried out for adults and children in India.39–42 46–49 A pivotal phase III study conducted on 398 adults demonstrated a cure rate of 94% (95% CI 91% to 97%) in the miltefosine arm. Phase IV studies involving 704 adults and 428 children were conducted in India and in Bangladesh (cure rates were of 82% overall50 and 72%, respectively51). Though trials involving miltefosine were still ongoing for CL52 53 and VL-HIV co-infection,54 in 2005—when the elimination initiative was launched—miltefosine was considered a game changer in the VL control strategy.22 46 55 The short time taken to bring the drug to market illustrates the efficiency of PPP in miltefosine’s R&D process: clinical trials started in 1996, and in 2002 the drug received approval to treat VL in India and in 2003 it was in the market (see figure 1). Oral administration (enabling straightforward management within primary care), gave rise to hopes for VL elimination.24 56
Milestones in miltefosine’s journey. R&D, research and development; VL, visceral leishmaniasis.
However, implementation of miltefosine treatment faces certain challenges. Miltefosine’s reproductive toxicity requires women of childbearing age to avoid pregnancy during, and for at least 3 months after treatment.57 The required pretreatment pregnancy screening and contraceptive cover severely hindered roll-out through primary care services in resource-limited settings. Gastrointestinal problems are common adverse events: up to 62% patients report vomiting/diarrhoea, although self-limiting.31 40 47 When the drug is self-administered, even mild adverse events may compromise adherence to a full regimen. Transient elevation of hepatic transaminases and mild renal dysfunction affect up to 10%–15% of patients.50 In phase IV trials in India and Bangladesh enrolling ~1000 patients, each recorded one death possibly related to the gastrointestinal side effects of miltefosine.50 51 Adverse events are thus common and need to be managed accordingly.
The difficulty in complying with a twice daily, 1 month treatment course,58 and a long half-life,59 all concur to make miltefosine monotherapy vulnerable to emerging drug resistance. The potential for resistance became a major concern in India when the drug was sold in private pharmacies and patients resorted to shorter courses due to affordability issues. India, therefore, restricted miltefosine provision to the public sector from 2008 onwards.27 60 Preserving efficacy of this valuable drug is crucial, and approaches such as directly observed treatment (DOT) and miltefosine use in combination regimens were thus recommended.61 After being used as a monotherapy for over a decade, miltefosine effectiveness reportedly declined: in India, 7% of patients with VL on DOT relapsed within 6 months;62 and in Nepal, the relapse rate was 20% for patients within 12 months on a self-administration schedule.63 This high failure rate, at least in the paediatric populations, was partly attributed to drug underexposure in paediatric populations at the recommended dose.64 65
Meanwhile, other treatment regimens were developed for VL66–68 and in 2014 the single-dose liposomal amphotericin B was rolled out through the elimination initiative replacing miltefosine monotherapy.69 Nevertheless, miltefosine remains an important drug in leishmaniasis therapy, as a companion in combination regimens, or in VL/HIV co-infected patients who require rotating multiple regimens. The spectrum of indications for miltefosine increased over time, currently covering VL caused by Leishmania donovani and postkala-azar dermal leishmaniasis in Asia, CL caused by Leishmania Viannia (Leishmania braziliensis, Leishmania guyanensis, Leishmania panamensis) and mucocutaneous leishmaniasis (MCL) caused by L. braziliensis.69 70 For CL and MCL, miltefosine is a useful alternative for use in paediatric populations (>2 years old) where existing treatment regimens prove insufficient.71 72
Postlicensure access to miltefosine: the early years and current status
Registration
Miltefosine was initially registered in India and Germany. Later, it has been approved for treatment of VL in Nepal, and for both VL and CL in Argentina, Bangladesh, Bolivia, Colombia, Ecuador, Guatemala, Honduras, Mexico, Pakistan, Paraguay, Peru, Israel and the USA—though some licenses may have lapsed and not been renewed by the company. Miltefosine received an orphan drug designation in the European Union in 200273 and the USA in 2006.74 WHO included the drug in its EML in 2011,25 26 underlining its public health importance.
Cost
Affordability is a critical issue for medicines developed to treat a poverty-related disease. An economic analysis has shown that for miltefosine to be an effective public health tool, the drug should cost no more than US$50–60 per treatment.45 75 The initial agreements, in the form of a memorandum of understanding between WHO and Asta Medica in 1995 provided the framework to ensure availability and affordability of the drug (see figure 1). The company was allowed to market the drug in the private sector but had to make it available at a preferential price within the public sector in all developing countries, conditional on the free provision of the drug to patients. The agreements stated that this preferential price should allow the company to recover the production cost plus a modest mark-up, while setting the price for the private sector would remain under the company’s control.
But, as the negotiation for the preferential price took years, miltefosine was at first only available in the private pharmacies in India at a cost of US$150–200 per treatment.27 This price is three to four times higher than the preferential one and well beyond the means of the majority of patients with VL, who had to pay out of pocket. The situation improved when preferential pricing was put in place, and after miltefosine was restricted to the public sector in India. Based on the initial agreement, the price of an adult treatment varied between €45–54 (US$54–64) depending on order quantity, at the time set at minimum 75 000 capsules.76 In the 2004 application for inclusion in WHO EML, the price quoted by Zentaris (Asta Medica spin-off acquired by Aeterna in 2002, later became Aeterna Zentaris in 2004—see figure 1 for complete chronology of ownership changes) was €80–300 for full adult treatment, the former for use in developing countries and the latter for the private sector.77 However, the preferential price has gradually increased over time, and for a period, it was only applicable when buying a full batch or 200 000 capsules (equivalent to 3500 treatment courses), a challenge for control programmes in countries like Nepal or Bangladesh with lower case numbers. Paladin (the owner company in 2008–2014, see figure 1) expressed in its 2010 application to WHO EML that price would not be a barrier,25 yet the conditions that need to be met for the preferential price were often unclear. The pricing structure provided by the supplier was not transparent: between 2009 and 2014, the price obtained by a non- governmental organisation (NGO) operating in endemic countries reached €250. Currently, the preferential price, according to Knight Therapeutics, sits between US$120 and US$160 per course, although there is no longer an obligation for minimum quantity (see table 1).
Price for one full adult course of miltefosine treatment
In Europe, the drug is only registered in Germany with one course costs €3000–12 000 (US$3500–14 000).45 Several access initiatives had been in place: in 2003, the company agreed to supply miltefosine for treating leishmania under special conditions for NGOs through a German medical aid organisation.78 Compassionate access programmes also exist for special cases, for example, VL/HIV co-infected patients,79 although many clinicians may be unaware. In the USA, a full drug course is in the range of US$17 000 (for 28 capsules, while a patient weighing >45 kg would need 50 mg thrice daily, amounting to 84 capsules)80 which health insurance is unlikely to cover.81 When used for treating free-living amoebas such as Acanthamoeba keratitis, miltefosine costs have reached US$48 000.82
Availability
Table 2 gives an overview of the main availability issues by region. The situation is indeed diverse. In the Indian subcontinent, frequent shortages of miltefosine have been reported by healthcare providers.28 Small-scale donations made possible by Paladin (see figure 1) did not solve the underlying problems. Obstacles to securing supply include bureaucratic, rigid tender mechanisms for public procurement; inadequate delivery systems; lack of buffer stock and difficulties in forecasting demand, as well as the long production lead time at the manufacturer. The minimum order quantities that were imposed by the company to be eligible for preferential prices for public or not-for-profit sectors seem to play a role, nonetheless. Earlier requirements to purchase a minimum of a full batch were not always compatible with the needs of the procurers (eg, for second-line treatment or clinical trials). The requirement thus had led to oversupply and wastage as the shelf life is limited, while substantial amounts of miltefosine expired in the manufacturer’s warehouse and had to be destroyed. Moreover, the global availability of miltefosine has been mostly depending on a single source. The ownership rights have been retained by the private company and have been exchanged over the years through business mergers and acquisitions (see figure 1). The change of companies for miltefosine has led to delays in delivering the drug on time.
Overview of miltefosine access issues by region
Since 2016, Knight owns worldwide rights to Impavido (miltefosine) related to its sale and distribution in all countries other than the USA.83 84 There, it was initially available through the Centres for Disease Control and Prevention and since 2015 after being approved for leishmaniasis by FDA, through Knight’s licensee, Profounda (figure 1). Currently, to say that the drug is freely available in the global market is an overstatement. Entities that need miltefosine have to approach Knight directly and negotiate, with little scope of collective action. Even in the Indian subcontinent where miltefosine is no longer first-line treatment, the medicine is still sorely needed for an alternative regimen, used in combination with paromomycin or liposomal amphotericin B (AmBisome)—and for treatment of HIV/VL. There are no accurate data on how many patients were treated with miltefosine since it was registered for VL. However, from 2008 to 2014, 163 000 VL cases were reported in India alone.85 The majority of these patients were supposedly treated with miltefosine.
Miltefosine is considered as a valuable compound in the field of leishmaniasis and beyond, thus several trials are still ongoing. However, no change in the pricing structure is foreseeable in the near future. More frustratingly, the US$125 million earned by Knight for registering miltefosine in 2014 in the USA, did not have any impact on the problematic access in developing countries, despite advocacy efforts by the civic societies.86
What are the lessons?
Miltefosine represents a major therapeutic advance for the treatment of leishmaniasis, with possible use against other pathogens. The drug’s development is a clear success story of a partnership between WHO, a private company and strongly motivated clinical researchers in endemic countries that proved that drug development for neglected diseases by PPPs is a viable model (figure 1).87–89 However, to date, access to miltefosine is limited, even in a context where preferential pricing should apply, and the manufacturer still has a de facto monopoly of a drug as the only quality-assured source. Based on miltefosine’s development history, we present policy recommendations for the wider drug development context and eventually narrow the train of our focus on practical suggestions to improve access to miltefosine for leishmaniasis.
One of the main lessons learnt is that miltefosine’s availability has been affected by the multiple changes in the ownership rights (as shown in figure 1) which resulted in changing distribution or marketing licenses for different subsidiaries over time.90–92 The agreement between WHO/TDR and the initial company—drafted to ensure continuous supply at an affordable price for public health use— could not be enforced with the company’s later successors. The case for needing a stronger agreement to ensure access in the postapproval phase is compelling, especially with the expansion of the PPP model for drug development, through organisations like the Drugs for Neglected Diseases initiative, Medicine for Malaria Venture and other entities.
Product development partnerships should set goals beyond mere registration of an NTD drug in endemic countries.93 94 Pharmaceutical or biotech companies targeting neglected diseases seem to operate a niche business model,45 seeking profits from both public and private markets in tiered pricing mechanisms. Tiered or differential pricing structure has been argued as a rational way of funding drug or vaccine availability in endemic resource-poor countries if effective access is indeed provided.95 However, sustained access under preferential pricing may not spontaneously yield robust market mechanisms for demand. Underlying PPP agreements must, therefore, include detailed and transparent provisions for sustained access, including pricing structures and frameworks for monitoring and enforcement.93 96 The absence of these structures and frameworks was a critical factor in the miltefosine journey. Furthermore, deployment strategies for new NTD drugs should also include long-term pharmacovigilance and feasibility studies for various contexts.
Another lesson is that some current incentive mechanisms meant to enhance R&D for NTDs seem to defeat their purpose. In 2014, the US FDA approved miltefosine registration for leishmaniasis, and Knight Therapeutics—which had acquired the rights to the drug the same year—was granted a reward: the tropical disease priority review voucher (PRV).97 98 PRV is enacted since 2007 to facilitate the development of drugs for NTDs. If a sponsor achieves approval for a new chemical entity that constitutes a significant improvement for one of the listed tropical diseases, the sponsor receives a PRV which can be used for priority review of any subsequent new drug or biologic under development.99 100 The voucher is transferable, and its value has been estimated to be up to US$350 million101.
While the voucher is meant to stimulate R&D for NTD drugs, the overall impact of the programme has yet to be established.102 103 In the case of miltefosine, as a drug co-developed with public money and already licensed in key countries, the lucrative incentive seems misplaced.104 Knight Therapeutics subsequently sold its PRV to Gilead for US$125 million,105 yet no improvements in miltefosine pricing or access in global markets have been seen so far.86 We suggest that preconditions on PRVs should stipulate that applicants seek regulatory approval of the drug in endemic countries, and demonstrate appropriate access strategies.103 106
Miltefosine is not the only leishmaniasis drug produced by a single manufacturer. In the long run, competitors or generic producers might help to secure supply and to stabilise prices. Miltefosine is no longer under patent protection, but generic manufacturers would need time or support to enter the market. It is worth noting that shrinking sales volume, as the number of VL cases decreases following elimination efforts on the Indian subcontinent, may deter potential producers. Nevertheless, as this is the the only oral treatment with potential for additional clinical indications within larger disease groups, efforts to ensure there are more quality-assured producers should continue. The addition of miltefosine to WHO’s invitation of expressions of interest for NTD prequalifications in 2017, is a step in the right direction.107
Several areas need to be addressed to overcome key access barriers to miltefosine (see table 3). Reducing access barriers to a life-saving drug needs a strong and sustained political commitment from the public sector, governments and global actors alike, supported by coherent policies. International coordinated procurement by multilateral organisations or advance market commitments should be sought to ensure miltefosine’s availability in the short term. In this regard, ensuring sufficient buffer or rotating stock at the regional level seems reasonable, if all stakeholders can reach a consensus. More transparent manufacturing timelines could help to avoid shortages, along with the better consolidation of forecast and orders. In the longer run, miltefosine registration in endemic countries needs to be reviewed and pursued. The inaccessibility of miltefosine should not be taken for granted, thus advocacy must continue. The current monopolistic situation must be challenged, hence encouraging new potential producers to enter the market would be beneficial. Harmonised actions to protect access to an essential public health tool, such as miltefosine, must be provided by the global public policy.
Summary of miltefosine access barriers and strategies to address them
Conclusion
The miltefosine story demonstrated the complexity of providing access to a promising NTD drug. Regrettably, apart from being a success story in R&D, the miltefosine journey embodies many flaws along the pathway from drug development to end user, and we observed issues of affordability and availability at global and country levels. Anticipated public health impact was hindered, as access barriers at different levels were not overcome. Strategies to expand access to an NTD drug thus must address affordability as a key obstacle, along with supply-side strategies that assure availability. Benefits of publicly funded medical research should be made broadly accessible to patients—neglect and imbalance should not be the end of the story.
Acknowledgments
The authors thank Els Torreele and Piero Olliaro for their critical reading of the manuscript, and Margriet den Boer, Koert Ritmeijer and Jose Postigo for their support. The authors also thank and Sarah Venis, Patricia Kahn, Kristien Cloots, Evelien Paessens; and Barbara Nasto for her expertise and enthusiasm.
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- 89.↵
- 90.↵
- 91.↵
- 92.↵
- 93.↵
- 94.↵
- 95.↵
- 96.↵
- 97.↵
- 98.↵
- 99.↵
- 100.↵
- 101.↵
- 102.↵
- 103.↵
- 104.↵
- 105.↵
- 106.↵
- 107.↵
- 108.
- 109.
- 110.
- 111.
Footnotes
Handling editor Seye Abimbola
Contributors TS, JP and MB conceived the paper. TS wrote the first draft. TS, JP, MB approved the final script.
Funding This study has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 642609.
Competing interests None declared.
Patient consent Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement No additional data are available.