Article Text
Abstract
Objective To compare late-preterm infants (33–36 weeks) with term infants (≥37 weeks) on incidence of morbidities in the first 3 years of life and healthcare costs during the first 2 years of life and third year of life.
Methods Administrative health records of live infants born between January 1, 1997, and December 31, 2000 with 3 years follow-up data (N=35733) were linked. First, diagnoses of morbidities were compared between late-preterm and term infants using Cox's proportional hazards models. Healthcare costs expressed as mean total costs and cost ratios, accrued following initial hospital discharge after birth, were also examined.
Results The three most common reasons for hospitalisation in late-preterm and term infants were acute bronchitis, otitis media and pneumonia. The most frequent reasons for physician visits included acute upper respiratory infections, otitis media and bronchiolitis. The highest HR were detected for chronic bronchitis 1.64 (1.13–2.39), hearing loss 1.56 (1.14–2.15) and bacterial diseases 1.28 (1.09–1.49). The mean total cost for late-preterm infants during the first 2 years of life was $2568 CAD compared with $1285 CAD for term infants, cost ratio =1.99 (95% CI 1.90 to 2.09). In the third year of life, the cost ratio reduced to 1.46 (95% CI 1.39 to 1.54).
Conclusions Late-preterm infants are at higher risk of specific morbidities compared with term infants. Their mean total costs fall from almost double that of term infants during the first 2 years of life, to just 46% greater in the third year of life.
Statistics from Altmetric.com
Introduction
Recent increases in premature births have been observed in many industrialised countries.1 Whether real or apparent, higher rates of multiple births, changes in the measurement of gestational age (GA)2 and advances in neonatology are potential contributor. Better access to neonatal care and improved high-risk obstetrical care have also led to mortality reductions in premature newborns.1 In Canada, the proportion of infants born at less than 37 gestational weeks increased from 6.6%3 in 1991 to 7.7% in 20034; in Quebec, the 2003 rate was 7.8%.4 Prematurity is responsible for 75% of neonatal morbidity5 ,6 resulting in considerable health service usage7;consequences extend beyond the neonatal period and lead to significant costs for health service providers, parents and society. However, few studies have examined the direct medical costs of hospitalisation and community physician services of premature infants following initial hospital discharge after birth.8 ,9
What is known on this topic
Infants born very premature are known to experience greater morbidity and have higher health service costs in the first years of life.
Infants born late preterm, however, have not been extensively studied and are generally considered functionally full term.
What this study adds
Late-preterm infants also experience greater morbidity compared with term infants.
The health service costs they incur over the first 2 years of life are significantly higher than those of term infants.
The health service costs of late-preterm infants approach those of term infants by the third year of life.
Subgroups within the well-defined classification of premature infants remain poorly defined.10 Among these, an important but understudied subgroup are ‘late-preterm’ infants, often considered functionally full term based on their relative large size,11 yet are physiologically and metabolically immature.12 Compared with term infants, late-preterm infants experience higher rates of morbidity12 ,13 and rehospitalisation,14 and their diligent evaluation and monitoring have been emphasised.15
Premature infants may exhibit ‘catch-up’ or accelerated growth that allows them to match the growth of term infants. Such infants are expected to achieve a growth path within normal reference ranges by 2–3 years of age.16 It is thus conceivable that after first hospital discharge following birth, late-preterm infants would accrue higher healthcare costs in the first 2 years of life, after which their physical maturity would become comparable with term infants. Our objectives were to compare late-preterm infants, defined in this study as infants born between 33 and 36 weeks GA, with term infants (≥37 weeks GA) according to (1) the morbidities during the first 3 years of life and (2) incurred healthcare costs during the first 2 years and the third year of life.
Methods
Data sources
We conducted a retrospective cohort study using the Quebec Registry Pregnancy, established with linkage of three administrative databases: (1) Régie de l’Assurance Maladie du Québec (RAMQ); (2) MED-ECHO and (3) Institut de la Statistique du Québec (ISQ). The RAMQ provides medical coverage to all 7.8 million Quebec residents and pharmaceutical coverage to 43% (welfare recipients, individuals ≥65 years, individuals not insured by their employer or spouse's employer); 36% of women between 15 and 45 years of age are covered by the RAMQ drug plan.17 Data holdings in the RAMQ include (1) prospectively collected data on physician-based diagnoses (International Classification of Diseases, ninth revision (ICD-9))18 and visits to physicians and emergency departments (EDs) and (2) validated data on filled medications.17 ,19 The MED-ECHO database records all acute care hospitalisations, including length of gestation, defined from the first day of the last menstrual period to the end of pregnancy, and confirmed by ultrasound. Medical diagnoses data in MED-ECHO have demonstrated validity.20 The ISQ database contains demographic information on the mother, father and baby. ISQ data have been compared with medical charts and validated.21 Linkage between RAMQ and MED-ECHO databases was done using individuals' numéro d’assurance maladie, a unique provincial personal identifier; linkage between RAMQ and ISQ databases was done using the mothers' and babies' names and birthdates. RAMQ, MED-ECHO and ISQ data in the Quebec Pregnancy Registry were available for all pregnancies between 1 January 1997 and 31 December 2000 that were covered by the RAMQ drug plan for at least 12 months before and during pregnancy. Pregnant women insured by the RAMQ drug plan have been shown to be of lower socioeconomic status but comparable with those insured by private insurance plans in terms of comorbidity profiles, use of prescribed and non-prescribed medications, and health service usage.17
Study population
We identified live infants born to mothers with at least 3 years of follow-up data after birth defined by continuous RAMQ medication coverage; with 6 years of available data, eligible infants would have been born within the first 3 years of data holdings. Infants were identified as late preterm (33 to 36 weeks GA) or term (≥37 weeks GA). We defined three time periods: first 3 years of life (years 0–3), first 2 years of life (years 0–2) and third year of life (years 2–3).
Morbidities
Diseases of interest included respiratory diseases, infectious diseases, mental disorders and diseases of the sense organs, nervous and circulatory system, defined using International Classification of Diseases (ICD-9) codes (shown in results tables). We recorded the first occurrence of diagnostic codes of interest during the first 3 years of life, capturing diagnoses made inhospital or during visits to community physicians.
Health service costs and usage
Direct health service costs were determined from the perspective of the provincial health insurance system. Expenses incurred by hospitals are indirectly covered by the Quebec Ministry of Health through an allocated annual budget. Physician services offered in out-patient clinics or inhospital are directly reimbursed by the RAMQ. We considered four cost components – inpatient hospital, ED, community physician visits and medications – and obtained information on quantities of resource used (eg, number of days spent in hospital). Clinical speciality-based per diem costs for hospitalisations were based on average costs representing bed and nursing costs in five greater Montreal hospitals22 and were as follows: paediatrics ($357), neonatology special care ($544), intensive care ($788), medicine ($222), paediatric psychiatry ($378) and ED ($137). For all other specialities, we applied the per diem cost of paediatric care. The cost of a physician visit was the reimbursed amount as recorded in the RAMQ database while medication costs comprised the cost of the medicine and professional fees minus patient co-pay, also recorded in RAMQ. All unit costs were expressed in Canadian dollars (price date, 2003).
Costs were calculated for the three time periods of interest, following initial hospitalisation after birth. In our administrative databases, subjects are tracked by their unique personal identifier. For newborns, there is usually a delay between birth and assignment of this identifier and it is often not possible to detect early events such as the precise date of first discharge from hospital after birth. In the absence of such information, the first discharge after birth was considered to have taken place at the 36th postconceptional week on the assumption that premature infants will remain in neonatal intensive care units until close to term to allow sufficient physiological maturation.23 Babies born after 36 weeks gestation were assumed to have been discharged after 48 h, following Society of Obstetricians and Gynaecologists of Canada guidelines.24
Statistical analysis
Cox's proportional hazards models were used to compare morbidities during the first 3 years of life in late-preterm and term infants. Multivariable models were adjusted for maternal age, marital status, number of years of schooling, RAMQ insurance status, place of residence, maternal diabetes, asthma, gestational hypertension, infant gender and birth weight.
Medians for total duration of hospitalisations, ED visits, number of physician visits and prescriptions were calculated as well as mean costs for each health service component and for all services combined. The ratio of mean total healthcare cost was computed since reporting relative costs may be more generalisable than absolute estimates.25 ,26 Costs were compared using the non-parametric Wilcoxon test, often used in data – such as cost data – with skewed distributions.27 Because this test compares differences in location between two distributions (medians) and the primary comparator of interest in cost analysis is the mean, we also applied a bootstrap method with 1000 replications to derive the bootstrap mean and 95% CI for total costs.27 ,28 The ratio of mean total costs was estimated by applying a generalised linear model specifying a γ distribution and log-link function.26 We conducted sensitivity analysis of discharge after birth by adding 48 h to the 36 weeks of gestation to evaluate how assuming a 2-day hospital stay for preterm and term infants will affect results. All analyses were performed using SAS (SAS Institute, Cary, North Carolina, USA).
Ethics approval was obtained from CHU Sainte Justine Research Ethics Board and Commission for Access to Information of Quebec.
Results
Overall 35 733 infants comprising 2051 late-preterm (5.70%) and 33 682 term infants (94.30%) met inclusion criteria. Table 1 compares the characteristics of infants (and mothers) in the two study groups.
Characteristics of mothers and infants according to late-preterm and term status
Adjusted HR (aHR) for the first diagnoses of morbidities during the first 3 years of life are shown in table 2. Diseases with significantly higher aHRs in late-preterm infants were respiratory disease (chronic bronchitis, 1.64 (95% CI 1.13 to 2.39)), infectious diseases (bacterial diseases, 1.28 (95% CI 1.09 to 1.49)) and hearing loss (1.56 (95% CI 1.14 to 2.15)).
Morbidity in the first 3 years of life for late-preterm infants compared with term infants
Table 3 summarises the most common primary diagnoses made inhospital and during community physicians' visits during the first 3 years of life. Within hospital, the proportion of late-preterm infants with diagnoses made for common diseases was higher than term infants. An opposite trend was observed for diagnoses of common diseases made during visits to community physicians.
Common reasons for hospital and community physician visits during first 3 years of life according to late-preterm and term status
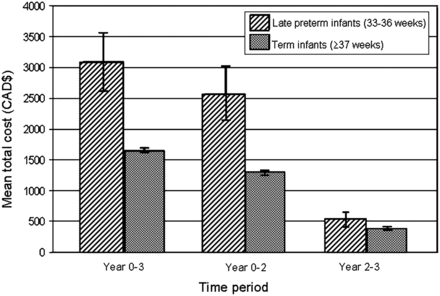
Costs associated with each type of health service, stratified according to the three study time periods, are shown in table 4. With the exception of ED visits during the third year of life, late-preterm infants had significantly higher usage and costs for all other health services. When we considered the total health service costs during the first 2 years of life, the costs for late-preterm infants was a factor of 1.99 (95% CI 1.90 to 2.09) over those for term infants. In the third year of life, this ratio reduced to 1.46 (95% CI 1.39 to 1.54). Figure 1 illustrates mean total costs for late-preterm and term infants for the three study time periods. There is a reduction in the difference in mean total cost between late-preterm and term infants from the first 2 years of life to the third year of life. Sensitivity analysis of discharge after birth assuming a 2-day hospital stay for preterm and term infants did not materially change results (data not shown).
Mean total healthcare cost* for late-preterm infants, and for term infants over the 3 years complete follow-up (years 0–3), first 2 years of life (years 0–2) and third year of life (years 2–3).
*Costs captured between 1997 and 2003.
Healthcare costs and health service usage according to late-preterm and term status
Discussion
This population-based study demonstrates that late-preterm infants had significantly higher risk for respiratory disease, infectious disease and hearing loss. A cost increase by a factor of almost 2 in the first 2 years of life and almost 1.5 in the third year of life was detected for late-preterm infants over term infants.
Our study provides insight into the medium term risks of diseases after initial discharge from hospital for late-preterm infants, adding to current literature which has focused on short-term outcomes during initial hospitalisation after birth.12 It also adds to limited literature on health service costs of late-preterm infants, specifically considering the first 3 years of life, and additionally stratifying costs for the first 2 years and third year of life.
We acknowledge limitations of our costing procedure. Speciality per diem costs were used as they most accurately represent the average cost of hospitalisation in specific departments. While this is superior to using generic costs of a bed day inhospital, it does not capture cost differences that may exist between patients with specific morbidities, potentially leading to underestimation of true hospitalisation cost. Nevertheless, this approach accurately captures relative costs between late-preterm and term infants. Furthermore, left truncation of costs at 36 weeks postconceptional age and cost enumeration until the third birthday allowed a standard period of data collection.
Sensitivity analyses on inpatient costs (the component with greatest uncertainty) would not have altered the ratio of costs between late-preterm and term infants, and we did not conduct such analyses. Reporting relative cost ratios under these circumstances provides valuable information beyond reporting absolute figures, since absolute costs could vary widely between health services and according to costing methodologies, and relative costs are more stable.25 While changes in costs over the 6-year study period may have occurred, cost data for events such as physician visits reflected current cost at the time of the event. It is possible that changes in the rate of preterm births reflecting an increase over time coupled with increasing unit costs over time may partly contribute to cost increases seen between late-preterm and term infants. However, it is unlikely that any such increase in late-preterm birth rates over the 3-year period in which all babies were born would have been of sufficient magnitude to affect our results.
Study strengths and limitations deserve comment. Linked administrative health data provided a large population-based cohort with complete follow-up data on hospitalisations, ED and physician visits and medication use. It was possible to investigate the incidence of a range of medical outcomes together with potential confounding variables. However, studies using administrative data are vulnerable to diagnostic uncertainty and use of administrative diagnostic codes to define morbidities may potentially lead to misclassification of diagnosis. Diagnoses recorded by ICD-9 codes are sometimes non-specific and provide no information on severity; information on other important confounders such as maternal weight gain, smoking, alcohol, drug abuse or race/ethnicity were lacking. Events recorded as hospitalisations in our study included inpatient admissions and day clinics while those recorded as ED visits did not capture events which resulted in subsequent hospitalisations. Therefore, rates presented may represent marginally higher or lower rates, in the former and latter cases, respectively. Our study population comprised (the babies of) mothers who were recipients of social assistance or workers without access to private medication insurance plans, representing a less affluent subset of Quebec's population, thus potentially limiting generalisability of our findings. However, we have previously shown that pregnant women insured by the RAMQ drug plan and those insured by private drug insurance plans have comparable comorbidity profiles and similar use of healthcare services such as physician visits and hospitalisations suggesting that costs would be similar as well.17 Our study period was limited to 3 years of follow-up after initial hospitalisation after birth. Given the delay in assigning unique identifiers to infants, we were not able to detect early events that occurred during this initial hospitalisation. Furthermore, certain conditions associated with preterm birth such as developmental problems may not be diagnosed until later in childhood. Investigations of secondary reasons for increased costs in preterm infants, such as being born into larger families and the risks of infection transmission, were beyond our scope and remain subjects for future research.
Consequences of prematurity may be far reaching and a societal perspective may be most ideal to capture the full impact. Our study was limited to the provincial health insurance system's perspective. Other studies of prematurity have demonstrated other consequences that are related to non-medical costs including travel for doctor visits and loss of earnings.29
Overall, this study suggests that late-preterm infants should not be considered as functionally full term and may require greater clinical attention than term infants especially during the first 2 years of life. Late-preterm infants have higher morbidity; however, their mean total healthcare costs fell from almost double that of term infants during the first 2 years of life to 46% in the third year of life, suggesting diminishing use of health services with increasing age.
References
Footnotes
Funding This study was supported by Les Fonds de la Recherche en Santé du Québec (FRSQ), the Réseau Québécois de recherche sur l’usage des médicaments (RQRUM) and the Réseau FRSQ for the well-being of children.
Competing interests None.
Ethics approval CHU Sainte Justine Research Ethics Board and Commission for Access to Information of Quebec.
Provenance and peer review Not commissioned; externally peer reviewed.
Linked Articles
- Fantoms
- Corrections