-
PDF
- Split View
-
Views
-
Cite
Cite
Suman Rijal, Bart Ostyn, Surendra Uranw, Keshav Rai, Narayan Raj Bhattarai, Thomas P. C. Dorlo, Jos H. Beijnen, Manu Vanaerschot, Saskia Decuypere, Subodh S. Dhakal, Murari Lal Das, Prahlad Karki, Rupa Singh, Marleen Boelaert, Jean-Claude Dujardin, Increasing Failure of Miltefosine in the Treatment of Kala-azar in Nepal and the Potential Role of Parasite Drug Resistance, Reinfection, or Noncompliance, Clinical Infectious Diseases, Volume 56, Issue 11, 1 June 2013, Pages 1530–1538, https://doi.org/10.1093/cid/cit102
Close - Share Icon Share
Abstract
Background. Miltefosine (MIL), the only oral drug for visceral leishmaniasis (VL), is currently the first-line therapy in the VL elimination program of the Indian subcontinent. Given the paucity of anti-VL drugs and the looming threat of resistance, there is an obvious need for close monitoring of clinical efficacy of MIL.
Methods. In a cohort study of 120 VL patients treated with MIL in Nepal, we monitored the clinical outcomes up to 12 months after completion of therapy and explored the potential role of drug compliance, parasite drug resistance, and reinfection.
Results. The initial cure rate was 95.8% (95% confidence interval [CI], 92.2−99.4) and the relapse rate at 6 and 12 months was 10.8% (95% CI, 5.2−16.4) and 20.0% (95% CI, 12.8−27.2) , respectively. No significant clinical risk factors of relapse apart from age <12 years were found. Parasite fingerprints of pretreatment and relapse bone marrow isolates within 8 patients were similar, suggesting that clinical relapses were not due to reinfection with a new strain. The mean promastigote MIL susceptibility (50% inhibitory concentration) of isolates from definite cures was similar to that of relapses. Although more tolerant strains were observed, parasite resistance, as currently measured, is thus not likely involved in MIL treatment failure. Moreover, MIL blood levels at the end of treatment were similar in cured and relapsed patients.
Conclusions. Relapse in one-fifth of the MIL-treated patients observed in our study is an alarming signal for the VL elimination campaign, urging for further review and cohort monitoring.
Visceral leishmaniasis (VL), or kala-azar, is endemic in the Indian subcontinent, affecting the Gangetic plains of Bangladesh, India, and Nepal. The annual VL incidence in this focus is estimated at 160 000–315 000 cases, or 80% of the worldwide VL burden [1]. In the Indian subcontinent, VL is due to Leishmania donovani; the transmission cycle is mostly anthroponotic, with the sand fly Phlebotomus argentipes as the unique reported vector. Recent breakthroughs as an oral drug (miltefosine [MIL]) and a rapid diagnostic test (RDT; the rk39 immunochromatographic test) were among the factors leading the 3 governments in the region to launch a VL elimination initiative [2]. MIL soon replaced sodium stibogluconate (SSG) as first-line therapy for VL [3, 4] as increasing treatment failure was reported [5, 6]. Although MIL showed excellent efficacy in phase 3 clinical trials almost a decade ago [7], there was always an apprehension of emergence of resistance due to its long elimination half-life, the possible noncompliance to oral therapy, and the anthroponotic transmission cycle [8, 9]. A recent report indicated a slightly decreased efficacy after a decade of MIL use in Bihar, India [10].
Surveillance of clinical efficacy of anti-VL drugs is not well standardized, in contrast to malaria [11]. By convention, an assessment 6 months after treatment is used in clinical trials as primary endpoint for the assessment of drug efficacy, but this endpoint is not routinely captured in the national control program. Failure of therapy in VL may be observed on the last day of treatment (also called nonresponse) or as relapse in the months after treatment. True relapses need to be distinguished from reinfections: this requires parasite fingerprinting at the onset of treatment and at the time of relapse, which is complicated by the important homogeneity of L. donovani populations in the Indian subcontinent [12–14]. Other, more likely reasons of therapy failure in VL are immunosuppression in the host (eg, human immunodeficiency virus [HIV] coinfection), clinical features (eg, disease progression or severity) [15], low quality of drug [16], low patient adherence, or drug resistance of the infecting parasite, which has not yet been documented for MIL in the Indian subcontinent. Given the paucity of anti-VL drugs, and the looming threat of resistance, there is an obvious need for monitoring carefully the clinical efficacy of MIL.
We launched a prospective cohort study in VL patients treated with MIL in Nepal and followed them up for up to 12 months after completion of therapy to document clinical outcomes. Drug compliance, parasite drug resistance, reinfection, and a series of clinical parameters were assessed, and their potential role in treatment failure was explored.
METHODS
This prospective study was conducted at the B. P. Koirala Institute of Health Sciences, a university hospital in eastern Nepal, from October 2008 to April 2011 in the framework of the KALADRUG-R project (http://www.leishrisk.net/kaladrug). All patients suspected to have VL (fever of ≥2 weeks’ duration with clinical splenomegaly) were admitted for a complete diagnostic workup, and patients ≥2 years of age with parasitologically proven VL were enrolled in the study after obtaining written informed consent from the patient or, in the case of children, from the parents or guardian.
Clinicoepidemiological information was recorded in a case record form. Investigations included complete blood count and chemistry, rapid diagnostic test for malaria, and HIV serology (Supplementary Data). Diagnosis of VL was confirmed by demonstrating Leishman-Donovan (LD) bodies in a Giemsa-stained bone marrow aspirate or spleen aspirate, if the former was negative. Aspirates were inoculated and placed in culture for further identification. VL patients were treated with MIL (Paladin Labs Inc, Montreal, Canada) as per the national guidelines (individuals aged ≥12 years weighing >25 kg: 100 mg daily; those weighing <25 kg: 50 mg daily; children aged 2–11 years: 2.5 mg/kg daily, for 28 days). Clinical outcomes were assessed at end of treatment (EOT), and at 3, 6 and 12 months after treatment using standard case definitions of treatment outcomes defined by the World Health Organization [17] (Table 1). Cases with recurrence of clinical signs were subjected to repeat smear examination. Compliance to the oral MIL treatment was assessed through questionnaires and MIL concentration analysis on end-of-treatment blood samples [18]. In case of death occurring during the follow-up period, verbal autopsy through family interview was done by a clinical doctor, to assess possible relation with VL or the treatment. We constructed Kaplan-Meier survival graphs and used Poisson regression to calculate incidence rate ratios of relapse in relation to different exposures. All exposures with a P value < .1 after univariate analysis were tested in a multivariate model. We used a backward elimination strategy, with probability for removal set at .05.
Case Definitions for the Outcome Recording of Visceral Leishmaniasis Patients
| Outcome . | Case Definition . |
|---|---|
| Early treatment outcomes | |
| Initial cure | Treatment completed, clinical improvement (absence of fever, regression of enlarged spleen + return of appetite and/or gain in body weight), and a negative smear at the end of MIL therapy. |
| Nonresponse | Signs and symptoms of VL persist + a positive smear at 28 days of MIL therapy. |
| Defaulter | VL patients who did not complete the 28-day treatment regimen of MIL and/or did not present for assessment after treatment. |
| Side effect–related switch | Side effects requiring MIL stop and change of treatment. |
| Death | Any death, whether or not related to KA. |
| Late treatment outcomes | |
| Definite cure | VL case with initial cure and no clinical signs (fever, or increase in spleen size since last visit), 12 months after completion of therapy. |
| Relapse | VL case with initial cure but with reappearance of clinical symptoms and/or signs along with smear positive for LD bodies during the 12-month follow-up. |
| Lost to follow-up | VL patient who completed therapy but who did not present/could not be traced for assessment at 12 months after treatment. |
| Death | Any death, whether or not related to KA. |
| Outcome . | Case Definition . |
|---|---|
| Early treatment outcomes | |
| Initial cure | Treatment completed, clinical improvement (absence of fever, regression of enlarged spleen + return of appetite and/or gain in body weight), and a negative smear at the end of MIL therapy. |
| Nonresponse | Signs and symptoms of VL persist + a positive smear at 28 days of MIL therapy. |
| Defaulter | VL patients who did not complete the 28-day treatment regimen of MIL and/or did not present for assessment after treatment. |
| Side effect–related switch | Side effects requiring MIL stop and change of treatment. |
| Death | Any death, whether or not related to KA. |
| Late treatment outcomes | |
| Definite cure | VL case with initial cure and no clinical signs (fever, or increase in spleen size since last visit), 12 months after completion of therapy. |
| Relapse | VL case with initial cure but with reappearance of clinical symptoms and/or signs along with smear positive for LD bodies during the 12-month follow-up. |
| Lost to follow-up | VL patient who completed therapy but who did not present/could not be traced for assessment at 12 months after treatment. |
| Death | Any death, whether or not related to KA. |
Treatment failure includes both nonresponse and relapse.
Abbreviations: KA, kala-azar; LD, Leishman-Donovan; MIL, miltefosine; VL, visceral leishmaniasis.
Case Definitions for the Outcome Recording of Visceral Leishmaniasis Patients
| Outcome . | Case Definition . |
|---|---|
| Early treatment outcomes | |
| Initial cure | Treatment completed, clinical improvement (absence of fever, regression of enlarged spleen + return of appetite and/or gain in body weight), and a negative smear at the end of MIL therapy. |
| Nonresponse | Signs and symptoms of VL persist + a positive smear at 28 days of MIL therapy. |
| Defaulter | VL patients who did not complete the 28-day treatment regimen of MIL and/or did not present for assessment after treatment. |
| Side effect–related switch | Side effects requiring MIL stop and change of treatment. |
| Death | Any death, whether or not related to KA. |
| Late treatment outcomes | |
| Definite cure | VL case with initial cure and no clinical signs (fever, or increase in spleen size since last visit), 12 months after completion of therapy. |
| Relapse | VL case with initial cure but with reappearance of clinical symptoms and/or signs along with smear positive for LD bodies during the 12-month follow-up. |
| Lost to follow-up | VL patient who completed therapy but who did not present/could not be traced for assessment at 12 months after treatment. |
| Death | Any death, whether or not related to KA. |
| Outcome . | Case Definition . |
|---|---|
| Early treatment outcomes | |
| Initial cure | Treatment completed, clinical improvement (absence of fever, regression of enlarged spleen + return of appetite and/or gain in body weight), and a negative smear at the end of MIL therapy. |
| Nonresponse | Signs and symptoms of VL persist + a positive smear at 28 days of MIL therapy. |
| Defaulter | VL patients who did not complete the 28-day treatment regimen of MIL and/or did not present for assessment after treatment. |
| Side effect–related switch | Side effects requiring MIL stop and change of treatment. |
| Death | Any death, whether or not related to KA. |
| Late treatment outcomes | |
| Definite cure | VL case with initial cure and no clinical signs (fever, or increase in spleen size since last visit), 12 months after completion of therapy. |
| Relapse | VL case with initial cure but with reappearance of clinical symptoms and/or signs along with smear positive for LD bodies during the 12-month follow-up. |
| Lost to follow-up | VL patient who completed therapy but who did not present/could not be traced for assessment at 12 months after treatment. |
| Death | Any death, whether or not related to KA. |
Treatment failure includes both nonresponse and relapse.
Abbreviations: KA, kala-azar; LD, Leishman-Donovan; MIL, miltefosine; VL, visceral leishmaniasis.
To test for reinfection, paired parasite isolates (pre-and postrelapse) were analyzed through kinetoplast DNA (kDNA) fingerprinting to discern relapse from reinfection. The obtained fingerprint patterns were analyzed using Bionumerics software version 5.10 (Applied Maths, Ghent, Belgium); a dendrogram was constructed using the unweighted pair group method with arithmetic mean to visualize the molecular similarity between the genotypes.
Parasite susceptibility was assessed by a previously validated promastigote assay [19]. Results were exported to Graph Pad Prism 5 (GraphPad Software) to calculate the 50% inhibitory concentration (IC50) using a sigmoidal dose-response model with variable slope, and results were statistically analyzed with a Mann-Whitney U test. Other details on testing and methodology are described in the Supplementary Data.
The ethics committee of the Nepal Health Research Council, Kathmandu, and the corresponding bodies at Antwerp University, Belgium, reviewed and approved the study protocol. Informed written consent was obtained from each patient or their guardian for those <18 years of age. All the patients and caretakers/parents had the study purpose explained to them in local language.
RESULTS
Clinical Results
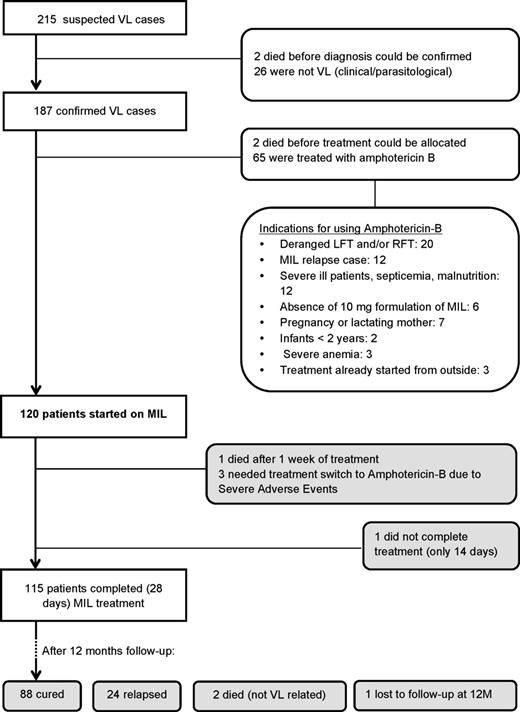
From May 2009 to April 2011, 217 patients with suspected VL were enrolled, of whom 187 were proven to have VL. The proportion of pediatric cases (<12 years) and women of childbearing age were 27.8% and 23.0%, respectively. Two patients (1.1%) were HIV positive. Amphotericin B was given in one-third (35.1%) of the cohort for reasons of contraindications, nonavailability of 10-mg MIL capsules, or previous history of MIL treatment followed by relapse (Figure 1). One hundred twenty patients were started on MIL (intention to treat) for whom a complete 12-month follow-up record was available in all but 1. Three patients required a switch to second-line treatment due to Common Toxicity Criteria grade III hepatotoxicity, 1 patient stopped his treatment after 14 days, and 1 died. In the 12 months after follow-up, 2 deaths occurred due to non-VL related causes according to verbal autopsy.
Recruitment, treatment, and follow-up of visceral leishmaniasis patients. Abbreviations: LFT, liver function tests; MIL, miltefosine; RFT, renal function test; VL, visceral leishmaniasis.
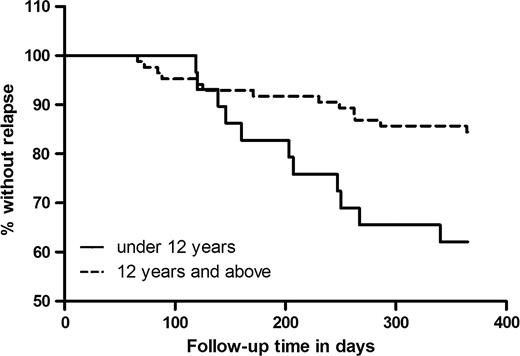
Outcome of therapy is shown in Table 2. The initial cure rate was 95.8% (95% confidence interval [CI], 92.2−99.4). In the following months, 24 patients who were initially cured presented with signs of relapse, confirmed through bone marrow smears. Relapse rate at 6 months was 10.8% (95% CI, 5.2−16.4) and 20.0% at 12 months (95% CI, 12.8−27.2). Table 3 shows evolution in clinical and biological variables at start of treatment, at EOT (ie, when cured) and at time of relapse, in the 24 relapse cases, As shown in Figure 2, relapse was most common among children (<12 years of age) and continued to occur beyond the commonly used 6-month follow-up period. Out of 24 documented relapses, 12 occurred >6 months after initial cure. Only age <12 years was significantly associated with relapse (Incidence Risk Ratio 2.43 [95% CI, 1.09–5.42], Table 4).
Treatment Outcomes at End of Treatment, at 6 Months, and at 12 Months After Miltefosine Treatment
| Outcome . | No. of Patients(n = 120) . | Cure Rate . | Cumulative Relapse Rate . |
|---|---|---|---|
| End of treatment | |||
| Treatment completed and cureda | 115 | 95.8% | |
| Deaths | 1 | ||
| Treatment-related switch | 3 | ||
| Incomplete treatment | 1 | ||
| After 6 mo | |||
| Still curedb | 99 | 82.5% | |
| Lost to follow-up | 1 | ||
| Extra deaths | 2 | ||
| Relapses | 13 | 10.8% | |
| After 12 mo | |||
| Still curedb | 88 | 73.3% | |
| Lost to follow-up | 1 | ||
| Extra relapses | 11 | ||
| Total number of relapsed | 24 | 20.0% | |
| Outcome . | No. of Patients(n = 120) . | Cure Rate . | Cumulative Relapse Rate . |
|---|---|---|---|
| End of treatment | |||
| Treatment completed and cureda | 115 | 95.8% | |
| Deaths | 1 | ||
| Treatment-related switch | 3 | ||
| Incomplete treatment | 1 | ||
| After 6 mo | |||
| Still curedb | 99 | 82.5% | |
| Lost to follow-up | 1 | ||
| Extra deaths | 2 | ||
| Relapses | 13 | 10.8% | |
| After 12 mo | |||
| Still curedb | 88 | 73.3% | |
| Lost to follow-up | 1 | ||
| Extra relapses | 11 | ||
| Total number of relapsed | 24 | 20.0% | |
a Definition of cure at end of treatment: clinical improvement and bone marrow aspiration smear negative.
b Definition of cure at 6 and 12 months after treatment: absence of signs and symptoms of visceral leishmaniasis.
Treatment Outcomes at End of Treatment, at 6 Months, and at 12 Months After Miltefosine Treatment
| Outcome . | No. of Patients(n = 120) . | Cure Rate . | Cumulative Relapse Rate . |
|---|---|---|---|
| End of treatment | |||
| Treatment completed and cureda | 115 | 95.8% | |
| Deaths | 1 | ||
| Treatment-related switch | 3 | ||
| Incomplete treatment | 1 | ||
| After 6 mo | |||
| Still curedb | 99 | 82.5% | |
| Lost to follow-up | 1 | ||
| Extra deaths | 2 | ||
| Relapses | 13 | 10.8% | |
| After 12 mo | |||
| Still curedb | 88 | 73.3% | |
| Lost to follow-up | 1 | ||
| Extra relapses | 11 | ||
| Total number of relapsed | 24 | 20.0% | |
| Outcome . | No. of Patients(n = 120) . | Cure Rate . | Cumulative Relapse Rate . |
|---|---|---|---|
| End of treatment | |||
| Treatment completed and cureda | 115 | 95.8% | |
| Deaths | 1 | ||
| Treatment-related switch | 3 | ||
| Incomplete treatment | 1 | ||
| After 6 mo | |||
| Still curedb | 99 | 82.5% | |
| Lost to follow-up | 1 | ||
| Extra deaths | 2 | ||
| Relapses | 13 | 10.8% | |
| After 12 mo | |||
| Still curedb | 88 | 73.3% | |
| Lost to follow-up | 1 | ||
| Extra relapses | 11 | ||
| Total number of relapsed | 24 | 20.0% | |
a Definition of cure at end of treatment: clinical improvement and bone marrow aspiration smear negative.
b Definition of cure at 6 and 12 months after treatment: absence of signs and symptoms of visceral leishmaniasis.
Evolution of Clinical and Laboratory Data (at Baseline; on Day 29, and at Time of Relapse) in the Patients Who Relapsed (n = 24)
| Variable . | Baseline . | Day 29 . | Changes From Baseline to Day 29 . | P Value . | Values at Time of Relapse . |
|---|---|---|---|---|---|
| Temperature, °C | 37.7 ± 0.8 | 36.7 ± 0.9 | −0.9 ± 0.8 | <.0001 | 37.4 ± 0.9 |
| Body weight, kg | 32.6 ± 15.5 | 33.6 ± 15.9 | 1.0 ± 1.6 | .005 | 34.5 ± 15.6 |
| Spleen size, cm below left costal margin | 6.0 ± 3.1 | 2.3 ± 2.3 | −3.8 ± 2.4 | <.0001 | 7.4 ± 3.2 |
| Liver size, cm below right costal margin | 2.3 ± 2.3 | 0.3 ± 0.9 | −1.9 ± 1.8 | <.0001 | 1.7 ± 1.9 |
| Hemoglobin, g/dL | 7.8 ± 2.1 | 9.0 ± 1.8 | 1.3 ± 1.9 | .004 | 8.0 ± 1.6 |
| WBC count, cells/mm3 | 3429.2 ± 2384.5 | 5787.5 ± 2286.3 | 2358.3 ± 2733.0 | .0003 | 3326 ± 1085 |
| Platelets, cells/mm3 | 148 400 ± 113815.1 | 193 791.7 ± 109886.5 | 45 391.7 ± 138 234.1 | .1213 | 125 173.9 ± 411 81.1 |
| Creatinine level, mg/dL | 0.9 ± 0.3 | 0.8 ± 0.2 | −0.05 ± 0.2 | .2281 | 0.8 ± 0.2 |
| Urea, mg/dL | 21.2 ± 7.3 | 19.3 ± 4.3 | −2.0 ± 6.7 | .165 | 21.2 ± 5.3 |
| SGOT, IU/mL | 50.8 ± 34.2 | 44.8 ± 17.0 | −6.0 ± 38.6 | .451 | 41.1 ± 22.7 |
| SGPT, IU/mL | 33.3 ± 21.8 | 44.6 ± 27.1 | 11.4 ± 26.5 | .0462 | 29.8 ± 17.7 |
| Variable . | Baseline . | Day 29 . | Changes From Baseline to Day 29 . | P Value . | Values at Time of Relapse . |
|---|---|---|---|---|---|
| Temperature, °C | 37.7 ± 0.8 | 36.7 ± 0.9 | −0.9 ± 0.8 | <.0001 | 37.4 ± 0.9 |
| Body weight, kg | 32.6 ± 15.5 | 33.6 ± 15.9 | 1.0 ± 1.6 | .005 | 34.5 ± 15.6 |
| Spleen size, cm below left costal margin | 6.0 ± 3.1 | 2.3 ± 2.3 | −3.8 ± 2.4 | <.0001 | 7.4 ± 3.2 |
| Liver size, cm below right costal margin | 2.3 ± 2.3 | 0.3 ± 0.9 | −1.9 ± 1.8 | <.0001 | 1.7 ± 1.9 |
| Hemoglobin, g/dL | 7.8 ± 2.1 | 9.0 ± 1.8 | 1.3 ± 1.9 | .004 | 8.0 ± 1.6 |
| WBC count, cells/mm3 | 3429.2 ± 2384.5 | 5787.5 ± 2286.3 | 2358.3 ± 2733.0 | .0003 | 3326 ± 1085 |
| Platelets, cells/mm3 | 148 400 ± 113815.1 | 193 791.7 ± 109886.5 | 45 391.7 ± 138 234.1 | .1213 | 125 173.9 ± 411 81.1 |
| Creatinine level, mg/dL | 0.9 ± 0.3 | 0.8 ± 0.2 | −0.05 ± 0.2 | .2281 | 0.8 ± 0.2 |
| Urea, mg/dL | 21.2 ± 7.3 | 19.3 ± 4.3 | −2.0 ± 6.7 | .165 | 21.2 ± 5.3 |
| SGOT, IU/mL | 50.8 ± 34.2 | 44.8 ± 17.0 | −6.0 ± 38.6 | .451 | 41.1 ± 22.7 |
| SGPT, IU/mL | 33.3 ± 21.8 | 44.6 ± 27.1 | 11.4 ± 26.5 | .0462 | 29.8 ± 17.7 |
Data are presented as mean± standard deviation.
Abbreviations: SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvate transaminase; WBC, white blood cell.
Evolution of Clinical and Laboratory Data (at Baseline; on Day 29, and at Time of Relapse) in the Patients Who Relapsed (n = 24)
| Variable . | Baseline . | Day 29 . | Changes From Baseline to Day 29 . | P Value . | Values at Time of Relapse . |
|---|---|---|---|---|---|
| Temperature, °C | 37.7 ± 0.8 | 36.7 ± 0.9 | −0.9 ± 0.8 | <.0001 | 37.4 ± 0.9 |
| Body weight, kg | 32.6 ± 15.5 | 33.6 ± 15.9 | 1.0 ± 1.6 | .005 | 34.5 ± 15.6 |
| Spleen size, cm below left costal margin | 6.0 ± 3.1 | 2.3 ± 2.3 | −3.8 ± 2.4 | <.0001 | 7.4 ± 3.2 |
| Liver size, cm below right costal margin | 2.3 ± 2.3 | 0.3 ± 0.9 | −1.9 ± 1.8 | <.0001 | 1.7 ± 1.9 |
| Hemoglobin, g/dL | 7.8 ± 2.1 | 9.0 ± 1.8 | 1.3 ± 1.9 | .004 | 8.0 ± 1.6 |
| WBC count, cells/mm3 | 3429.2 ± 2384.5 | 5787.5 ± 2286.3 | 2358.3 ± 2733.0 | .0003 | 3326 ± 1085 |
| Platelets, cells/mm3 | 148 400 ± 113815.1 | 193 791.7 ± 109886.5 | 45 391.7 ± 138 234.1 | .1213 | 125 173.9 ± 411 81.1 |
| Creatinine level, mg/dL | 0.9 ± 0.3 | 0.8 ± 0.2 | −0.05 ± 0.2 | .2281 | 0.8 ± 0.2 |
| Urea, mg/dL | 21.2 ± 7.3 | 19.3 ± 4.3 | −2.0 ± 6.7 | .165 | 21.2 ± 5.3 |
| SGOT, IU/mL | 50.8 ± 34.2 | 44.8 ± 17.0 | −6.0 ± 38.6 | .451 | 41.1 ± 22.7 |
| SGPT, IU/mL | 33.3 ± 21.8 | 44.6 ± 27.1 | 11.4 ± 26.5 | .0462 | 29.8 ± 17.7 |
| Variable . | Baseline . | Day 29 . | Changes From Baseline to Day 29 . | P Value . | Values at Time of Relapse . |
|---|---|---|---|---|---|
| Temperature, °C | 37.7 ± 0.8 | 36.7 ± 0.9 | −0.9 ± 0.8 | <.0001 | 37.4 ± 0.9 |
| Body weight, kg | 32.6 ± 15.5 | 33.6 ± 15.9 | 1.0 ± 1.6 | .005 | 34.5 ± 15.6 |
| Spleen size, cm below left costal margin | 6.0 ± 3.1 | 2.3 ± 2.3 | −3.8 ± 2.4 | <.0001 | 7.4 ± 3.2 |
| Liver size, cm below right costal margin | 2.3 ± 2.3 | 0.3 ± 0.9 | −1.9 ± 1.8 | <.0001 | 1.7 ± 1.9 |
| Hemoglobin, g/dL | 7.8 ± 2.1 | 9.0 ± 1.8 | 1.3 ± 1.9 | .004 | 8.0 ± 1.6 |
| WBC count, cells/mm3 | 3429.2 ± 2384.5 | 5787.5 ± 2286.3 | 2358.3 ± 2733.0 | .0003 | 3326 ± 1085 |
| Platelets, cells/mm3 | 148 400 ± 113815.1 | 193 791.7 ± 109886.5 | 45 391.7 ± 138 234.1 | .1213 | 125 173.9 ± 411 81.1 |
| Creatinine level, mg/dL | 0.9 ± 0.3 | 0.8 ± 0.2 | −0.05 ± 0.2 | .2281 | 0.8 ± 0.2 |
| Urea, mg/dL | 21.2 ± 7.3 | 19.3 ± 4.3 | −2.0 ± 6.7 | .165 | 21.2 ± 5.3 |
| SGOT, IU/mL | 50.8 ± 34.2 | 44.8 ± 17.0 | −6.0 ± 38.6 | .451 | 41.1 ± 22.7 |
| SGPT, IU/mL | 33.3 ± 21.8 | 44.6 ± 27.1 | 11.4 ± 26.5 | .0462 | 29.8 ± 17.7 |
Data are presented as mean± standard deviation.
Abbreviations: SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvate transaminase; WBC, white blood cell.
Risk Factors for Miltefosine Relapse (Poisson Analysis)
| Variables . | Relapse (n = 24) . | Cured (n = 96) . | Incidence Risk Ratio . | 95% CI . | P Value . |
|---|---|---|---|---|---|
| No. (%) . | No. (%) . | ||||
| Age group | |||||
| ≤12 y | 11 (45.8) | 20 (20.8) | 2.43 | 1.09–5.42 | .030 |
| >12 y | 13 (54.2) | 76 (79.2) | Referent | ||
| Sex | |||||
| Male | 17 (70.8) | 57 (59.4) | 1.51 | .63–3.64 | .359 |
| Female | 7 (29.2) | 39 (40.6) | Referent | ||
| Past VL treatment | |||||
| Yes | 5 (20.8) | 15 (15.6) | 1.24 | .46–3.32 | .668 |
| No | 19 (79.2) | 81 (84.4) | Referent | ||
| Baseline hemoglobin | |||||
| <7 gm/dL | 11 (45.8) | 26 (27.1) | 1.90 | .85–4.24 | .118 |
| ≥7 gm/dL | 13 (54.2) | 70 (72.9) | Referent | ||
| Duration of fever | |||||
| >10 wk | 9 (37.5) | 35 (36.5) | 1.04 | .45–2.37 | .932 |
| ≤10 wk | 15 (62.5) | 61 (63.5) | Referent | ||
| Baseline body mass index, kg/m2 | |||||
| Low, <18.5 | 16 (66.7) | 55 (57.3) | 1.41 | .59–3.4 | .436 |
| Normal, 18.5–24.5 | 8 (33.3) | 41 (42.7) | Referent | ||
| Variables . | Relapse (n = 24) . | Cured (n = 96) . | Incidence Risk Ratio . | 95% CI . | P Value . |
|---|---|---|---|---|---|
| No. (%) . | No. (%) . | ||||
| Age group | |||||
| ≤12 y | 11 (45.8) | 20 (20.8) | 2.43 | 1.09–5.42 | .030 |
| >12 y | 13 (54.2) | 76 (79.2) | Referent | ||
| Sex | |||||
| Male | 17 (70.8) | 57 (59.4) | 1.51 | .63–3.64 | .359 |
| Female | 7 (29.2) | 39 (40.6) | Referent | ||
| Past VL treatment | |||||
| Yes | 5 (20.8) | 15 (15.6) | 1.24 | .46–3.32 | .668 |
| No | 19 (79.2) | 81 (84.4) | Referent | ||
| Baseline hemoglobin | |||||
| <7 gm/dL | 11 (45.8) | 26 (27.1) | 1.90 | .85–4.24 | .118 |
| ≥7 gm/dL | 13 (54.2) | 70 (72.9) | Referent | ||
| Duration of fever | |||||
| >10 wk | 9 (37.5) | 35 (36.5) | 1.04 | .45–2.37 | .932 |
| ≤10 wk | 15 (62.5) | 61 (63.5) | Referent | ||
| Baseline body mass index, kg/m2 | |||||
| Low, <18.5 | 16 (66.7) | 55 (57.3) | 1.41 | .59–3.4 | .436 |
| Normal, 18.5–24.5 | 8 (33.3) | 41 (42.7) | Referent | ||
Abbreviations: CI, confidence interval; VL, visceral leishmaniasis.
Risk Factors for Miltefosine Relapse (Poisson Analysis)
| Variables . | Relapse (n = 24) . | Cured (n = 96) . | Incidence Risk Ratio . | 95% CI . | P Value . |
|---|---|---|---|---|---|
| No. (%) . | No. (%) . | ||||
| Age group | |||||
| ≤12 y | 11 (45.8) | 20 (20.8) | 2.43 | 1.09–5.42 | .030 |
| >12 y | 13 (54.2) | 76 (79.2) | Referent | ||
| Sex | |||||
| Male | 17 (70.8) | 57 (59.4) | 1.51 | .63–3.64 | .359 |
| Female | 7 (29.2) | 39 (40.6) | Referent | ||
| Past VL treatment | |||||
| Yes | 5 (20.8) | 15 (15.6) | 1.24 | .46–3.32 | .668 |
| No | 19 (79.2) | 81 (84.4) | Referent | ||
| Baseline hemoglobin | |||||
| <7 gm/dL | 11 (45.8) | 26 (27.1) | 1.90 | .85–4.24 | .118 |
| ≥7 gm/dL | 13 (54.2) | 70 (72.9) | Referent | ||
| Duration of fever | |||||
| >10 wk | 9 (37.5) | 35 (36.5) | 1.04 | .45–2.37 | .932 |
| ≤10 wk | 15 (62.5) | 61 (63.5) | Referent | ||
| Baseline body mass index, kg/m2 | |||||
| Low, <18.5 | 16 (66.7) | 55 (57.3) | 1.41 | .59–3.4 | .436 |
| Normal, 18.5–24.5 | 8 (33.3) | 41 (42.7) | Referent | ||
| Variables . | Relapse (n = 24) . | Cured (n = 96) . | Incidence Risk Ratio . | 95% CI . | P Value . |
|---|---|---|---|---|---|
| No. (%) . | No. (%) . | ||||
| Age group | |||||
| ≤12 y | 11 (45.8) | 20 (20.8) | 2.43 | 1.09–5.42 | .030 |
| >12 y | 13 (54.2) | 76 (79.2) | Referent | ||
| Sex | |||||
| Male | 17 (70.8) | 57 (59.4) | 1.51 | .63–3.64 | .359 |
| Female | 7 (29.2) | 39 (40.6) | Referent | ||
| Past VL treatment | |||||
| Yes | 5 (20.8) | 15 (15.6) | 1.24 | .46–3.32 | .668 |
| No | 19 (79.2) | 81 (84.4) | Referent | ||
| Baseline hemoglobin | |||||
| <7 gm/dL | 11 (45.8) | 26 (27.1) | 1.90 | .85–4.24 | .118 |
| ≥7 gm/dL | 13 (54.2) | 70 (72.9) | Referent | ||
| Duration of fever | |||||
| >10 wk | 9 (37.5) | 35 (36.5) | 1.04 | .45–2.37 | .932 |
| ≤10 wk | 15 (62.5) | 61 (63.5) | Referent | ||
| Baseline body mass index, kg/m2 | |||||
| Low, <18.5 | 16 (66.7) | 55 (57.3) | 1.41 | .59–3.4 | .436 |
| Normal, 18.5–24.5 | 8 (33.3) | 41 (42.7) | Referent | ||
Abbreviations: CI, confidence interval; VL, visceral leishmaniasis.
Relapse Versus Reinfection
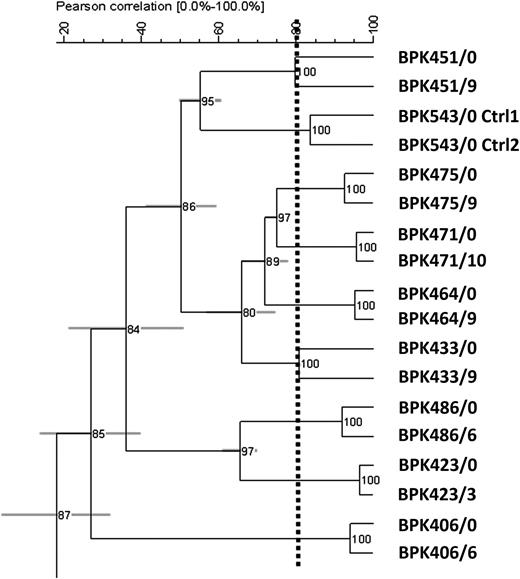
Parasite fingerprinting was directly applied to bone marrow aspirates taken at the onset of treatment and at the time of relapse in 8 paired samples. The resulting kDNA restriction patterns were used for cluster analysis (Figure 3). Two replicas from the reference strain BPK543/0 were included to test the robustness of the obtained clusters. Based on the standard deviation of the branches, and consistency of clustering, 9 kDNA fingerprint types were recognized among the 18 samples (8 pairs and 2 replicas) analyzed. Remarkably, they corresponded to the 8 patients’ bone marrow samples and the replicates of the reference strain. In other words, (1) the parasites present in these 9 samples were all different, hereby validating the discriminatory power of our method on the present set of parasites, and (2) the genotype of the parasites was identical at the onset of treatment and at the time of clinical relapse. This strongly suggests that clinical relapses were not due to reinfection with a new strain.
UPGMA dendogram based on fingerprint densitometric curve similarities from kinetoplast DNA PCR-RFLP with HaeIII, applied on the 8 pair of clinical samples and experimental replicas. Horizontal lines indicate the standard deviation of each branching position as deduced from comparing dendogram and curve similarities. The dotted line denoted the interexperiment repeatability threshold: only groups diverging at the left of the line define robust genotypes, while splits occurring at the right may represent experimental variation. Pearson correlation (0–100%).
Parasite Drug Susceptibility
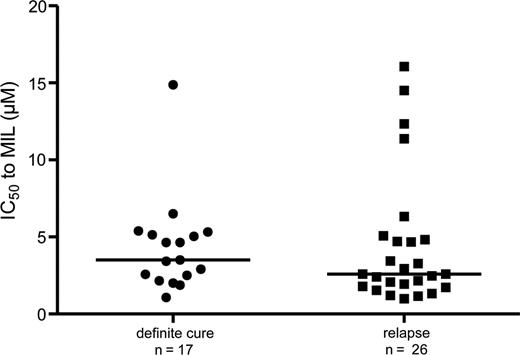
Using a previously validated promastigote assay [19], we assessed the parasites’ susceptibility to MIL and compared (1) between isolates from definite cures and relapses, taking the pretreatment isolate into account when >1 is available of the same patient, (2) between pre- and posttreatment isolates in general, and (3) between pre- and posttreatment isolates of the same patient when available. The median promastigote MIL susceptibility (and 25th–75th percentiles [IC50]) of isolates from definite cures (n = 17) was 4.328 µM (2.338–5.230 µM) compared to 2.595 µM (1.780–4.885 µM) for isolates from relapses (n = 26) (Figure 4). There was no statistical difference between the 2 groups (P = .3265). When the susceptibility of all pretreatment isolates (n = 27) was compared to all posttreatment isolates (n = 18), again no significant differences were observed (P = .4943). Also the 2 pairs of isolates, that is, a pre- and posttreatment isolate of the same patient, showed a similar in vitro promastigote MIL susceptibility.
In vitro promastigote miltefosine (MIL) susceptibility of strains isolated from MIL definite cures and MIL relapses. No statistical significant difference between groups was found. Horizontal bars indicate the median. Abbreviations: IC50, 50% inhibitory concentration; MIL, miltefosine.
Treatment Compliance and Drug Exposure
The data in the case record forms regarding MIL intake and adherence of follow-up revealed that all patients received the correct dosing as per the national guidelines. All adhered to the scheduled visits during the treatment except for 1 patient who was contacted by telephone but confirmed completing the full 28 days of therapy. All patients treated with MIL completed the treatment, except for 5 of 120 (1 defaulted after 14 days, 3 switched to second-line treatment, and 1 died during treatment). Duration of treatment period varied from 28 to 36 days (mean, 29 days), and interruption of therapy of ≥2 days was observed in 3 patients. There was one outlier of 47 days due to alternate day treatment because of elevated liver tests.
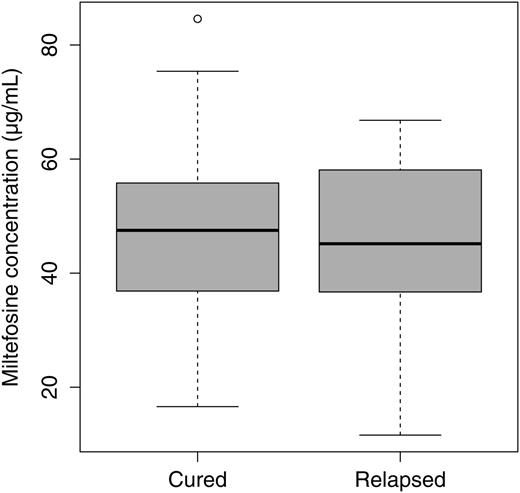
To assess and compare the exposure of patients to MIL, the accumulated MIL concentration at EOT, corresponding with the steady-state concentration, was analyzed. Only results for patients whose sampling time did not exceed >7 days before or beyond EOT were included for comparison. Finally, there were 64 included EOT MIL concentrations, from 48 cured and 16 relapsed cases representing 54.5% of cured and 66.6% of the relapses. As shown in Figure 5, there was no significant difference in MIL concentration at EOT between cured and relapsed patients. The mean EOT MIL whole blood concentrations were 46.7 µg/mL (SD, 15 µg/mL) and 44.5 µg/mL (SD, 16.6 µg/mL) for the cured and relapsed patients, respectively (P = .762). Relapsed patients who were included in this pharmacokinetic comparison were younger than cured patients, although not significantly (mean age, 25 years vs 19 years, respectively; P = .209). In support of the long shelf-life of miltefosine (4 years) and the confirmed reputed source of procurement of miltefosine (Paladin Labs), indeed no apparent degradation of MIL content could be demonstrated at the end of the project in a small sample of capsules (n = 3): the amount of active ingredient was within ±15% of the declared content.
Comparison of miltefosine concentration at the end of treatment (± 7 days) in cured (n = 48) versus relapsed (n = 16) visceral leishmaniasis patients. No significant difference between groups was found.
DISCUSSION
In this prospective cohort study conducted 5 years after the introduction of MIL in Nepal, we observed relapse in one-fifth of the MIL-treated patients. This treatment failure was not due to reinfection with a new strain, and none of the relapsing patients were HIV-positive. All patients with failure had initially responded well to the MIL treatment (initial cure rate of 95.8%), but relapsed in the months thereafter. Cure rate at 6 months after treatment was 82.5%, which dropped to 73.3% at 12 months after treatment. This is an important finding in view of the current convention of considering the 6-month cure rate as the final endpoint in clinical research. The final cure rate seen in our cohort is much lower than that observed in a similar study from India (90%) [10] but similar to cure rates from Bangladesh (85%) [20]; both studies assessed outcomes only up to 6 months. It is noteworthy that all MIL treatment failures here observed were due to relapses, contrasting with the situation encountered for SSG at the end of the SSG era, with many nonresponsive patients [15]. The reasons for therapy failure are multiple and can be related to the host, the drug, or the parasite, all of which were assessed in this study.
With respect to the host, we did not find any other significant risk factors or predictors of relapse apart from age <12 years. This parameter was not significant in a previous study on risk factors of SSG treatment failure of VL [15], contrasting with the case of American tegumentary leishmaniasis [21]. Possible differences in child immune response, drug pharmacokinetics, and exposure to antigens may affect outcome. Duration of fever (proxy of disease advancement) has been observed to be associated with increased treatment failure with SSG [15] but this was not seen with MIL.
With respect to the drug itself, no apparent degradation of the MIL content in the capsules during the project period could be identified. No significant difference in the mean EOT MIL concentration between cured and relapsed patients was identified. Nonetheless, large variability in MIL concentrations, and demonstrated relative underexposure of children compared to adults receiving the conventional MIL dosage [22], are factors possibly obscuring the effect of MIL exposure on the probability of outcome in this study. If adherence was inadequate, with a substantial number of missed doses, then this would have affected not only overall exposure but also the EOT concentrations due to the inherent accumulation of MIL until the last week of treatment [22]. The mean whole blood EOT MIL concentration measured here corresponds with historic pharmacokinetic data in Indian adults receiving a similar dosage (100 mg or approximately 2.5 mg/kg/day), that is, 46 µg/mL in whole blood versus 45 µg/mL in plasma [23]. The EOT MIL concentrations were measured for the majority of relapse cases and did not indicate a discrepancy with the self-reported compliance to therapy in this group.
With respect to the parasite itself, 2 parameters were assessed: (1) the genotype of paired samples in relapsing patients and (2) the in vitro susceptibility of isolates. For parasite fingerprinting, we applied a polymerase chain reaction–restriction fragment length polymorphism assay targeting kDNA mini-circles, which has shown to be resolute enough to distinguish strains in the Indian subcontinent, directly on clinical samples. Individuals who recover from a VL episode are reported to develop a cellular immune response protecting them from reinfection [24]. According to some authors, this protection would be life-long [25], precluding reinfection. The fingerprinting data observed in our study did not point at reinfection, as nearly identical genotypes were encountered in each patient at the onset of treatment and at time of relapse, while different strains were observed between the different patients. To assay Leishmania's drug susceptibility, parasite isolates are required and generally assays must be applied on intracellular amastigotes as for many drugs, promastigotes are intrinsically insensitive to the drug [26]. This is not the case for MIL, and a recent study validated the use of promastigotes for susceptibility assay by demonstrating a significant correlation between IC50 measured on both life stages [19]. Resistance to miltefosine has shown to be easily induced in vitro [27–29], but clear-cut natural MIL-resistant isolates have as yet not been identified in the field up to now, except for the recent report of acquired MIL resistance in a HIV-coinfected patient in France [30]. In the current study, when comparing isolates from patients with MIL-definite cure with those of patients with MIL relapse, both showed similar in vitro MIL susceptibilities, indicating that MIL relapse of these patients cannot be associated to the in vitro MIL-resistant phenotype of the infecting parasite as defined in our study. Outliers with an IC50 of approximately 15 µM were observed in strains isolated from both cured and relapsed patients, but these values are still significantly lower than those shown by in vitro–induced MIL-resistant promastigotes (±40 µM) or the recently observed natural MIL-resistant Leishmania infantum isolate from an Algerian HIV-coinfected patient in France [30]. In regard to the demonstrated in vitro susceptibility of MIL relapse isolates, the existence and role of in vivo reservoirs or sanctuary sites for the Leishmania parasite may be postulated. In these local reservoir sites, Leishmania parasites may have a lower exposure to the drug or exhibit different parasite dynamics compared to the systemic compartment. The existence of such reservoir sites, where in the long-term parasites eventually survive treatment, would be in line with the high initial cure rate and the observed systemic miltefosine exposure. In any case, the more tolerant parasites that were identified here and elsewhere [31] may constitute the first steps toward the development of full resistance in the future. Therefore, close epidemiological monitoring is required, which can be facilitated by simplified promastigote susceptibility tests as applied here.
The reduced effectiveness of miltefosine observed in our study is an alarming signal for the VL elimination campaign. Drug policies should be reviewed to achieve better cure rates and to protect the few available drugs. An excellent efficacy of single-dose liposomal amphotericin B and combination therapy was recently demonstrated in phase 3 trials [32, 33], and probably provide better therapeutic options for the VL elimination initiative than MIL monotherapy. There is also a need to strengthen pharmacovigilance and establish continuous monitoring of the effectiveness of drug regimens in the elimination program. Our findings point to the need for longer follow-up, at least in the Nepalese context, but possibly also in the other endemic areas.
Notes
Acknowledgments. We gratefully acknowledge Dr Epco Hasker (Department of Public Health, Institute of Tropical Medicine, Antwerp, Belgium) for his support in data analysis. We wish to express our gratitude to all VL patients for their active participation in the study. The authors are grateful to the medical doctors Saru Devkota, Kajan Ranabhaat, Richa Bhattarai, Romila Chimoriya, Rajan Sharma, Sunit Agarwal, and Janak Das for their active involvement in clinical management of VL patients. The authors are also grateful to Santosh Bastola, Ganesh Sah, Ichha Ghale, sisters, and staff of the Tropical Ward, B. P. Koirala Institute of Health Sciences, for their cooperation and kind support during the study period.
Financial support. This work was supported by the European Commission's Seventh Framework Program (Kaladrug-R project, grant 222895).
Disclaimer. The funder had no role in the study design, data collection and analysis, interpretation or reporting of this work, or the decision to submit the work for publication. All authors are independent of the funding source.
Author contributions. Design of the study: S. R., B. O., S. D., M. B., J. D. Data collection: S. R., S. U., K. R., N. R. B., S. S. D., M. L. D., P. K., R. S. Data analysis and interpretation: S. R., B. O., S. U., K. R., N. R. B., T. P. C. D., J. H. B., M. V., S. D., M. B., J. D. Writing of the manuscript: all authors.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.





Comments