Article Text
Abstract
Neonatal mortality remains a major global challenge. Most neonatal deaths occur in low-income countries, but it is estimated that over two-thirds of these deaths could be prevented if achievable interventions are scaled up. To date, initiatives have focused on community and obstetric interventions, and there has been limited simultaneous drive to improve neonatal care in the health facilities where the sick neonates are being referred. Few data exist on the process of implementing of neonatal care packages and their impact. Evidence-based guidelines for neonatal care in health facilities in low-resource settings and direction on how to achieve these standards of neonatal care are therefore urgently needed. We used the WHO-Recommended Quality of Care Framework to build a strategy for quality improvement of neonatal care in a busy government hospital in Eastern Uganda. Twelve key interventions were designed to improve infrastructure, equipment, protocols and training to provide two levels of neonatal care. We implemented this low-cost, hospital-based neonatal care package over an 18-month period. This data-driven analysis paper illustrates how simple changes in practice, provision of basic equipment and protocols, ongoing training and dedicated neonatal staff can reduce neonatal mortality substantially even without specialist equipment. Neonatal mortality decreased from 48% to 40% (P=0.25) after level 1 care was implemented and dropped further to 21% (P<0.01) with level 2 care. In our experience, a dramatic impact on neonatal mortality can be made through modest and cost-effective interventions. We recommend that stakeholders seeking to improve neonatal care in low-resource settings adopt a similar approach.
- neonatal care
- low resource setting
- Africa
- neonatal unit
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Key messages
What is already known about this topic?
Globally, neonatal mortality remains the major barrier to further reductions in the under-five mortality.
It is estimated that two-thirds of these neonatal deaths could be prevented if achievable interventions are scaled up.
Growing evidence suggests that by bundling effective neonatal health interventions together, cost-effective and successful reductions in neonatal mortality can be achieved.
What are the new findings?
Implementation of a low-cost hospital-based neonatal care package within an existing government healthcare system can have a significant impact on neonatal mortality.
Simple changes in practice, basic equipment, ongoing training and dedicated neonatal staff can reduce neonatal mortality even in the absence of specialist equipment.
Recommendations for policy
Limited additional funds are required to achieve level 1 neonatal care, and all district hospitals in low-income countries (LICs) should offer this level of neonatal care as a minimum.
Level 2 neonatal care requires a greater financial and personnel commitment to achieve; however, the benefits of such investment are strikingly clear, and such care should be provided by all regional hospitals in LICs.
Without implementing the basic elements of level 1 and 2 neonatal care, the addition of specialist neonatal equipment is futile.
Introduction
Globally, neonatal mortality remains a major barrier to reducing under-five mortality.1 Nearly all neonatal deaths occur in low-income countries (LICs), and thirty-nine per cent of them occur in sub-Saharan Africa.1 The leading causes of death are prematurity (28%), infections (26%) and intrapartum-related events (23%).2 It is estimated that over two-thirds of these deaths could be prevented if achievable interventions are scaled up.3–5 Growing evidence suggests that by bundling effective neonatal interventions together, cost-effective and successful reductions in mortality can be achieved.3 4
To date, initiatives to reduce neonatal mortality have focused on community, primary care and obstetric interventions. Such programmes have looked at improving antenatal care, essential newborn care, recognition of sick neonates and improving referral to health facilities. However, there has been limited simultaneous drive to improve the neonatal care available in the health facilities where the neonates are being referred. In many hospitals in LICs, neonates are nursed in paediatric wards without specialist neonatal care. Where hospital-based neonatal care has been implemented in LICs, significant impacts on mortality have been demonstrated.6–9 If the unacceptable number of neonatal deaths are to be reduced, in addition to improving community neonatal care and emergency care at the time of delivery, hospital-based neonatal care needs to be developed to ensure a full cycle of care. Some frameworks already exist to evaluate and improve neonatal care, but they focus primarily on obstetric-led neonatal emergency management and not on ongoing specialised neonatal care.10 11 Few data exist on the process of implementing facility-based neonatal care in low-resource settings and their impact.3 Evidence-based guidelines for neonatal care in government health facilities in low-resource settings and direction on how to achieve these standards are urgently needed. Neonatal care should be made available at every level, from health centres to regional hospitals, and guidelines should delineate the expected level of neonatal care for each level of healthcare facility.
We report the development and implementation of two novel levels of evidence-based and low-cost neonatal care and their associated impact on mortality in a government hospital in Eastern Uganda.
Developing a neonatal care programme
Mbale Regional Referral Hospital (MRRH) serves 4.5 million people in Eastern Uganda and has nearly 10 000 deliveries a year. It is the only hospital with specialists in the region and receives neonatal referrals from health facilities well beyond its catchment area. Until October 2014, MRRH had no dedicated neonatal care. Neonates were admitted to the paediatric ward, treated by medical staff with no specific neonatal training and nursed alongside older children. Neonatal mortality appeared to be high, but there was no substantive data to support this.
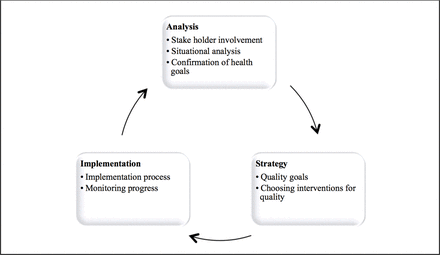
In August 2014, following the WHO-Recommended Quality of Care Framework (figure 1), a strategy for neonatal quality improvement was built.12 The quality improvement project was led by the paediatrician with the support of the hospital administration. A baseline analysis was carried out by the paediatrician to assess the existing standard of neonatal care, the number of neonatal admissions and their outcomes were recorded in a purpose-designed neonatal logbook (online supplementary appendix 1). Once established, this routine data collection continued throughout the quality improvement strategy. Through interviews and meetings with stakeholders including doctors, nurses, pharmacists, parents, hospital administrators and district health officials, interventions were chosen for quality improvement, and health goals were set. The final neonatal care programme (NCP) addressed 12 key areas (table 1) and was introduced in two stages as permitted by logistical and financial constraints. Before commencing the project, commitment was sought from the hospital administration to support the construction of the neonatal unit (NNU), supply the required neonatal medications and allocate adequate nurses and/or midwives to work specifically on the NNU.
Supplementary file 1
The 12 key interventions identified for development of a neonatal care programme
The WHO-Recommended Quality of Care Framework.9
We believe level 1 care (interventions 1–9) can be easily implemented alongside existing paediatric care. Level 1 care does not require specialist equipment, only a separate area within a paediatric ward and fundamental changes in clinical practice, which can be achieved through training. However, level 2 care (interventions 1–12) requires a separate neonatal ward, dedicated nurses with basic neonatal training, a paediatrician and investment in appropriate technology.
Interventions
Routine data collection on neonatal admissions
It was acknowledged that neonatal mortality was high, but as is all too often the case in LICs, accurate data were not available. Routine neonatal data collection was established prior to the implementation of level 1 care and was continued throughout the implementation of the NCP (online supplementary appendix 1). This allowed the burden of neonatal illness and the impact of the quality improvement strategy to be evaluated.
Regular monthly audit of neonatal admissions, outcomes and mortality
Recognising the importance of clinical audit, monthly joint neonatal and maternal mortality meetings were initiated.13 These meetings provided a forum for discussion and allowed ongoing identification of areas for quality improvement and continuing education of the staff.
Maternal education, involvement and empowerment (Mbale mother-centred model)
Traditionally, neonatal care requires a high nurse to patient ratio to provide effective care.14 In many LICs, this is not feasible, and in many instances, it is only a single nurse caring for all the neonates on the ward. In order to replicate one-on-one nursing, the ‘Mbale Mother-Centred Model’ was developed where mothers and attendants were empowered to undertake basic ‘nursing’ care including feeding. A maternal bed was provided adjacent to the neonatal cot to facilitate this. A trained nursing assistant gave daily teaching to the mothers to enable them to monitor axillary temperature and daily weights. The mothers were also taught about the eight danger signs, cord care, the practice and benefits of kangaroo care (KC), how to express breast milk and how to provide spoon-feeding or nasogastric tube feeding.15 Pictorial posters helped reinforce the daily teaching.
Neonates, particularly preterms, are at a high risk of hypothermia even in tropical climates, and it is a leading risk factor for neonatal mortality in LICs.16 17 The absence of continuous power, engineering support, maintenance and satisfactory disinfection makes the safe use of incubators challenging. The NNU room temperature varied from 25°C to 35°C depending on the time of day and season. There was no need therefore to warm the ambient temperature of the room, and thermoregulation was maintained by KC. KC has been proven to half the mortality of preterm infants (<2 kg) through improving thermoregulation, reducing sepsis and improving breastfeeding.18 KC also empowers mothers to care for their preterm at home, a vital tool when mothers cannot afford to spend long in hospital. The NCP focused on training neonatal staff about KC and implementing its use for preterm infants. This practice was continued for neonates on both oxygen and continuous positive airways pressure (CPAP). Mothers were encouraged to practice KC both in the hospital and after discharge.
Protocols and guidelines to aid neonatal case management
Neonatal guidelines were written to provide all staff with a simple framework to assess and manage neonates. When possible, evidence from LICs was used. If unavailable, evidence from middle-income or high-income countries (HICs) was used or adapted, but only when it was affordable, feasible and sustainable. The guidelines were separated into three main sections: emergency care, priority care and ongoing care. The priority care guidelines allowed staff to make a quick assessment of key problems and led them to the correct, immediate and lifesaving management. The ongoing care chapters provided simple but more detailed information on each diagnosis including risk factors, clinical presentation, investigations, emergency management and ongoing management.
Staff training in neonatology
Our 14 module Neonatal Care Training Course complemented the neonatal guidelines. The course was provided free of charge by the paediatrician, and procurement of teaching materials was supported by a non-governmental organisation (NGO). One 2-hour module was taught each week to minimise interference with limited staffing. Acknowledging that neonatal care needed to extend seamlessly into antenatal, perinatal and paediatric care, all staff in the hospital were invited to attend, particularly those working in areas with direct contact with neonates such as maternity and paediatrics. The course began at the inception of level 1 care, and 57 midwives, nurses and interns from MRRH attended. The training included practical sessions, interactive lectures and videos on the recognition and management of common neonatal problems: essential newborn care, neonatal resuscitation, danger signs, routine newborn examination, assessment and immediate management of the sick baby, neonatal infections, babies with difficult breathing, babies with difficult feeding, care of the small baby, care of the jaundice baby, perinatal asphyxia and case scenarios.
Guidelines and training in the administration of neonatal medications
Many medications have no specific preparation for neonates; therefore, the small doses required are often challenging to accurately achieve.19 A simplified formulary based on the British National Formulary for Children was designed for all the neonatal drugs used (figure 2, online supplementary appendix 2).20 A basic prescription chart was introduced to minimise prescription errors (online supplementary appendix 3). For all the staff attending Neonatal Care Training Course, teaching on the formulary, prescription chart, medication preparation and administration was delivered by the hospital pharmacist.
Supplementary file 2
Supplementary file 3
Neonatal medications used and an example page of the formulary. NS, normal saline.
Guidelines and training in the administration of neonatal feeds and intravenous fluids
In LICs, total parenteral nutrition is rarely available, but it is still vital to provide intravenous fluids while neonates establish enteral feeds. Protocols were created for 10% dextrose and neonatal fluid (0.18% saline and 8% dextrose). The protocols also focused on the volume of fluid to be given. Although continuous administration of fluids has key benefits of glucose and blood pressure homeostasis, this can only effectively be achieved by syringe drivers, which are often unavailable in LICs. Other similar settings have used two hourly boluses of intravenous fluid, which is challenging to administer and risks undulating blood pressure and hypoglycaemia.21 The NCP therefore implemented fluid administration using burettes in six hourly volumes. A simple fluid prescription chart was introduced to aid prescription and administration of fluids and feeds (online supplementary appendix 4). Training was given on preparing, calculating and administering intravenous fluids.
Supplementary file 4
Many sick and preterm neonates are unable to breastfeed, so mothers were asked to express breast milk two hourly into a clean dry cup. The cup and other equipment were stored in a clean plastic bucket to prevent contamination, and unused milk was discarded after each feed. Neonates who could suck but not safely swallow, such as preterms >1500 g, were fed using a spoon. Neonates who were unable to swallow safely, such as encephalopathic neonates and preterms <1500 g, used nasogastric tube feeding. All neonates achieved spoon-feeding before discharge.
Dedicated area for neonatal patients and improved infection control policies
During level 1, care neonates were admitted to the paediatric ward, which although common in LICs, was not ideal. Therefore, spatial delineation was made between the 16-bed neonatal area and the paediatric patients. An additional 8-bed side room was allocated for KC and was fitted with a sink for hand washing, a neonatal resuscitation area and an area for fluid and medication preparation. Vital equipment was installed on the paediatric ward; an electronic baby scale (SECA 354), digital axillary thermometers (OMRON Ecotemp Basic), tape measures, a neonatal stethoscope, a neonatal ambu-bag, size 0 and 1 face masks and penguin suction.
Neonatal follow-up clinic
Due to the financial and social constraints that are often faced in LICs, mothers are frequently unable to stay long in hospital. Services are needed to support early discharge and continuation of care as an outpatient. A weekly neonatal follow-up clinic was established to facilitate ongoing care of preterm neonates and neonates who had suffered more severe illnesses such as meningitis or hypoxic ischaemic encephalopathy (HIE). Neonates from both within and from outside the catchment area attended this clinic. Preterm infants were reviewed weekly until 1500 g and fortnightly until 2500 g. Infants were assessed for weight gain, head growth, development and signs of infection. Mothers were given ongoing support in feeding, medications, KC and immunisations, and any unwell neonates were readmitted.
Level 2 care
Dedicated NNU
Neonates, particularly preterms, are at a high risk of contracting infection. In HICs, neonates are admitted to a separate NNU, with strict infection control procedures. To address these key issues and to achieve level 2 care, a dedicated NNU was developed by partitioning off a section of the postnatal ward (figure 3). Multiple electric sockets were installed for equipment, and a power stabiliser was installed to minimise power surges and protect equipment. Sinks were fitted to facilitate hand washing and washing of equipment. A water-tank was installed to ensure a continuous water supply. The development of the NNU infrastructure costs <£2000 and was provided by the hospital administration.
The dedicated neonatal unit.
NNU admission criteria were inborn and outborn patients of all gestational ages up to a corrected postnatal age of 28 days. Neonates with skin infections and diarrhoea were not admitted. Lack of space, multiple visitors, overcrowding and lack of running water are but a few factors that make infection control challenging in LICs. Creation of a dedicated NNU addressed some of these issues. Visitors were not permitted, attendants and staff removed their shoes and washed their hands before entering and personal belongings were not allowed. Staff had uniforms that were only worn on the NNU, and a small changing area allowed attendants to change into a hospital gown before entering. A dedicated neonatal assistant cleaned the NNU two times per day. In addition, alcohol sanitiser was provided to cleanse hands and instruments between patients.
Specific beds were allocated to preterms to minimise their contact with infectious cases. A 5-bed high dependency area was located adjacent to the nurse’s station, where the sickest babies were monitored continuously using pulse oximetry (LifeBox). Neonatal bassinets and adjacent maternal beds were provided for each patient to facilitate the practice of KC and the Mbale Mother-Centred Care model.
Investment in appropriate technology for neonatal care
Neonatal care typically relies on a high level of technology such as incubators, ventilators, syringe drivers and CPAP. Such equipment is prohibitively expensive and requires a high-level of healthcare worker training, engineering support, maintenance and a reliable power supply. A number of companies have focused on designing low-cost, robust and simple equipment for low-resource settings. Through stakeholder meetings, key equipment was identified.
Oxygen saturations were monitored (LifeBox) two times per day to promote the responsible use of oxygen.22 23 Three oxygen concentrators (Diamedica) were installed, and flow splitters delivered 0–2.5 L/min to individual infants using nasal cannulae. Three CPAP machines (Diamedica) were used not only for their robust and low-cost nature but also for their ability to blend oxygen and air. This was vital if retinopathy was to be minimised. Phototherapy was provided by LED phototherapy machines (Brilliance) designed for low-resource settings.
Dedicated neonatal staff including a paediatrician and neonatal nurses
The NNU had a dedicated paediatrician and a neonatal clinical officer who led daily ward-rounds. These staff were permanent and did not rotate, they were key to providing continuity and training to the rotating nursing staff. The placement of the neonatal clinical officer was supported by an NGO. In settings like this where doctors are limited, pressure often falls on nursing staff. The NNU had six dedicated nurses and midwives, working 8-hour shifts, allowing one nurse to cover the ward at any time. The neonatal nurses were government nurses/midwives allocated by the hospital administration to work on the NNU.
There is currently no specific neonatal nursing training in Uganda, so the nurses attended the aforementioned local Neonatal Care Training Course and were given on-the-job training by the paediatrician and neonatal clinical officer to help them assess neonates, make simple diagnoses and initiate emergency and immediate management. They were trained in cannulation, venepuncture, capillary blood sampling, nasogastric tube insertion and lumbar punctures. In Uganda, nurses are normally rotated through different wards on an annual basis. During this study, the NNU experienced one rotation of nursing staff at which time two neonatal nurses remained and the new nurses underwent the same training as described above. Key to the success of this project was the presence of permanent staff to provide ongoing mentorship and training.
Evaluating the NCP
Over 18 months, 2890 neonates were admitted, with 240 (8%) in preintervention, 650 (22%) during level 1 and 2000 (69%) during the first year of level 2 (figure 4). Outcome measures were monitored continuously during the staged implementation. These included the number of admissions, overall mortality and disease specific mortality from the key diagnoses; prematurity, neonatal sepsis and HIE (figure 5). Continuous prospective data collection was established using a purpose-designed admission logbook (online supplementary appendix 1). Data were extracted monthly into Microsoft Office Excel. Ethical clearance for this study was granted by the MRRH Research and Ethics Committee. We used descriptive analysis and Χ2 test for independence in STATA V.14 to compare differences between the preintervention, level 1 care and level 2 care groups, with statistical significance at a P value <0.05. The construction of the contingency tables was based on proportions of reported mortality to reduce the denominator effect.
The number of neonatal admissions during the three study periods.
Clinical case definitions. HIE, hypoxic ischaemic encephalopathy.
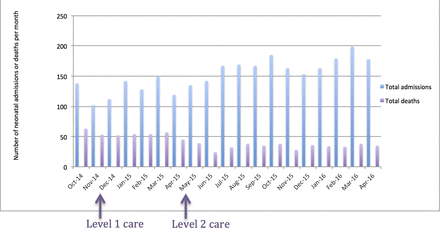
During the implementation, neonatal admissions doubled (figure 6). It is likely that when neonatal services were absent and perceived mortality was high, care-seeking behaviour was limited. Once healthcare workers and mothers became aware of a novel and successful service, the likelihood of care seeking increased. In addition, the training of the midwives and nurses on the labour and postnatal ward, through the Neonatal Care Training Course, helped improve the recognition of sick neonates and thereby improved their referral to NNU. This is consistent with observations in Ethiopia, where neonatal admissions increased threefold following the creation of the NNU.8
The monthly neonatal admissions and mortality from October 2014 until April 2016.
Reducing mortality
During the implementation, we registered an impressive decline in neonatal deaths (figure 6). Overall, neonatal inpatient mortality decreased from 48% to 40% (P=0.254) after level 1 care (table 2) and to 21% after level 2 (P<0.01).
Impact on overall mortality for each level of neonatal care implemented
There was a significant reduction in all three leading causes of inpatient neonatal mortality (table 3). Mortality due to prematurity decreased from 65% to 43% (P=0.002) with level 1 care and to 28% (P=0.027) with level 2 care. The mortality from neonatal sepsis reduced from 41% to 34% (P=0.307) and then to 8% (P<0.001) with level 1 and 2 care, respectively. Unexpectedly, deaths due to HIE increased with level 1 care from 44% to 67% (P=0.001), but significantly reduced with level 2 care (36%, P<0.001). Improved recognition and referral likely explains the increased mortality of neonates with HIE seen during level 1 care. Level 1 care showed effectiveness in averting death due to prematurity, and yet level 2 had broader substantial gain in reducing overall mortality. Therefore to have a significant impact on neonatal mortality, level 2 care is the minimal level that should be implemented in a referral hospital. Similar observations were made following improved infrastructure, equipment and clinical protocols in an NNU in Mozambique where the mortality rate for asphyxia (34% vs 19%), sepsis (39% vs 28%) and prematurity (43% vs 33%) decreased significantly.6 It is estimated that around one-quarter of neonatal deaths occur in the first day; therefore, emphasis on improving neonatal care needs to extend to the staff in the labour and postnatal ward to reduce neonatal mortality.2
Impact on disease specific mortality for each level of neonatal care implemented
Focusing on achievable, cost-effective and sustainable interventions
The belief that only advanced technology can reduce neonatal mortality seems unfounded and unconfirmed in our study. Focusing on achievable, cost-effective and sustainable interventions such as training, infection control, KC and appropriate feeding can dramatically reduce the number of neonatal deaths in LICs.4 Severe nursing shortages in LICs contribute to the high neonatal mortality. In the presence of low staff to patient ratios, appropriate training and involvement of the mothers has proved to reduce mortality and improve weight gain in low birth weight neonates compared with professional nursing in LICs.24 25 In MRRH, mothers were trained to monitor and care for their neonates regardless of birth weight. We believe this mother-centred model contributed significantly to the reduction in mortality and also decreased the workload on the nurses, which has the potential for saving both lives and costs. The introduction of KC at MRRH and the provision of neonatal resuscitation training for nearly all maternity and paediatric staff likely had a considerable impact on neonatal deaths.26–29
Focusing on improved provision of neonatal care in the paediatric department
The two levels of neonatal care described in this analysis are novel. Previous frameworks have focused primarily on the improvement of neonatal care through better provision of skilled care during childbirth, essential newborn care, basic and comprehensive emergency obstetric and newborn care interventions (BEmONC and CEmONC).10 11 These interventions focus heavily on the provision of routine care for well neonates and emergency life-saving interventions at delivery such as immediate exclusive breastfeeding, neonatal resuscitation and corticosteroids in preterm labour. What is still greatly needed is an evidence-based guideline on the continuation of neonatal care for sick and preterm neonates once they leave the delivery room. Merging labour ward interventions such as EmONC with an ongoing and complementary NCP similar to that described in this analysis has a huge potential for reduction in neonatal mortality and is something that should be explored. Without the concurrent development of dedicated neonatal services and programmes alongside improved emergency newborn care, the full potential of improved neonatal survival will not be achieved.
Limitations and strengths
This analysis had both limitations and strengths. First, we employed retrospective pre–post design, which cannot control for additional external changes; however, we were not aware of any other changes in the referral facilities, referral pathways or obstetric care during this period. Second, the preintervention data collection phase is short because it was not considered ethical to extend this period when interventions to reduce inpatient neonatal mortality had been identified and were available. Third, this study relied on routinely collected hospital data for analysis; therefore, detailed data were not available for comparison. This is however one of the first studies to report on the implementation of hospital-based neonatal care in a government hospital in an LIC and its impact on neonatal mortality.
Conclusion
The implementation of a low-cost hospital-based intervention package within the existing healthcare system is a clear example of how simple and achievable changes in practice, basic equipment, ongoing training together with dedicated neonatal staff can reduce neonatal mortality substantially even in the absence of specialist equipment. The involvement of hospital administrators and district health officials from the inception of the project and their commitment to improving the quality of neonatal care within the hospital was vital in the success of this project. Without the commitment and support from the administrators, this project would have been neither successful nor sustainable. All district government hospitals in LICs should be able to achieve level 1 care with minimal additional funds. Level 2 care requires greater financial and personnel commitment from the hospital; however, the benefits of such investment are strikingly clear. Integrating neonatal care into existing services is a promising intervention to reduce avoidable deaths with manageable additional cost. Without implementing these key elements, the addition of specialist equipment is futile. True effectiveness trials conducted at scale in health systems in similar settings through wide-scale implementation and evaluation of a similar evidence-based programme are needed.
Acknowledgments
The authors would like to thank the neonates and their families. They also thank the dedicated neonatal nurses, midwives, paediatric nurses and the obstetric team for the care of these neonates. The authors extend their thanks to the then hospital director, Dr Wanume Benon and the MRRH hospital administration team for their support in the development of the NNU. They also express their thanks to the equipment donations and support for the neonatal training course from Born on the Edge, a non-governmental organisation operating in the Mbale region.
References
Footnotes
Handling editor Seye Abimbola
Contributors KB, JI, AH-S, TO and PO-O initiated the project. All authors made substantial contribution in developing the strategy for quality improvement. KB led the implementation. KB, JI, SA, MK, ST, AS, TO and AH-S contributed to the implementation of this model. KB and FO wrote the statistical analysis plan, cleaned and analysed the data. KB drafted the manuscript. All authors have seen and approved the final manuscript.
Competing interests None declared.
Ethics approval Mbale Regional Referral Hospital research and ethics committee.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement No additional data are available.